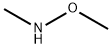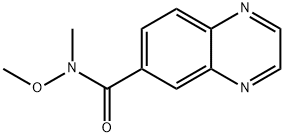
Quinoxaline-6-carboxylic acid methoxy-methyl-amide synthesis
- Product Name:Quinoxaline-6-carboxylic acid methoxy-methyl-amide
- CAS Number:875558-38-6
- Molecular formula:C11H11N3O2
- Molecular Weight:217.22
6925-00-4
164 suppliers
$23.00/1g
6638-79-5
569 suppliers
$6.00/25g

875558-38-6
19 suppliers
inquiry
Yield:875558-38-6 99%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in tetrahydrofuran;dichloromethane; for 12 h;Inert atmosphere;
Steps:
1.1A
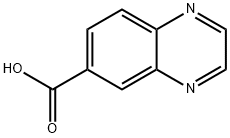
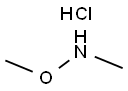
Example 12-(1,2-Dimethyl-3-oxo-5-quinoxalin-6-yl-2,3-dihydro-1H-pyrazol-4-yl)-benzonitrileStep 1A: Quinoxaline-6-carboxylic acid methoxy-methyl-amideA 2000 mL round bottomed flask was charged with 21.0 g (121 mmoles) of quinoxaline-6-carboxylic acid, 32.4 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (169 mmoles), 22.8 g of 1-hydroxybenzotriazole (169 mmoles), and 24.7 g of N,O-dimethylhydroxylamine hydrochloride (253 mmoles) under a nitrogen atmosphere. To this, 315 mL of tetrahydrofuran and 210 mL of dichloromethane were added. Next, 127 mL of triethylamine (729 mmoles) was charged, and the reaction was allowed to stir for 12 hours. After the reaction was complete, the contents of the flask were concentrated to 1/3 volume under vacuum, and 440 mL of water was added. The mixture was extracted with ethyl acetate (3×200 mL); the subsequent organic fractions were then washed with saturated sodium bicarbonate (1×200 mL) and dried over sodium sulfate. The final product (26.1 g; 99% yield) was obtained after removing the solvent under vacuum. The product was used without further purification.1H NMR 300 MHz (CDCl3) δ 3.35 (s, 3H), 3.49 (s, 3H), 7.97 (d, J=8.7 Hz, 1H), 8.05 (d, J=8.7 Hz, 1H), 8.36 (s, 1H), 8.81 (s, 1H).M/Z Theoretical: 217.09; M/Z+1 217.77.
References:
US2010/56505,2010,A1 Location in patent:Page/Page column 25

6638-79-5
569 suppliers
$6.00/25g
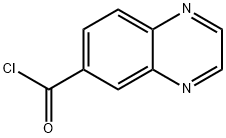
258503-93-4
38 suppliers
$44.00/100mg

875558-38-6
19 suppliers
inquiry

6925-00-4
164 suppliers
$23.00/1g

6638-79-5
569 suppliers
$6.00/25g
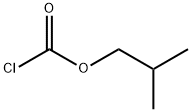
543-27-1
258 suppliers
$10.00/5g

875558-38-6
19 suppliers
inquiry