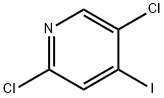
2,5-Dichloro-4-iodopyridine synthesis
- Product Name:2,5-Dichloro-4-iodopyridine
- CAS Number:796851-03-1
- Molecular formula:C5H2Cl2IN
- Molecular Weight:273.89
16110-09-1
458 suppliers
$5.00/1g

796851-03-1
131 suppliers
$19.00/250mg
Yield:796851-03-1 53%
Reaction Conditions:
Stage #1:2,5 dichloropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;2-Methylpentane at -78; for 1.5 h;Inert atmosphere;
Stage #2: with iodine in tetrahydrofuran;2-Methylpentane at -78;Inert atmosphere;
Steps:
3.01.a
a) A solution of 2,5-dichloropyridine (10 g, 67.57 mmol) in THF (17 mL) was added dropwise to a stirred solution of n-BuLi in isohexane (33.8 mL, 67.57 mmol) and diisopropylamine (9.63 mL, 67.57 mmol) in THF (68.0 mL) cooled to -780C, over a period of 1 hour under a nitrogen atmosphere. The resulting mixture was stirred at -780C for 30 minutes and then a solution of I2 (17.49 g, 68.92 mmol) in THF (17.0 mL) was added dropwise. The resulting solution was stirred at -78 0C for 1 hour and then quenched with water (75 mL) and allowed to warm to room temperature. The mixture was extracted with Et2O (3x 100 mL) and the combined organic layers were dried over MgSO4, and then evaporated. The residue was triturated with CH2Cl2 to give a solid which was dried under vacuum to afford 2,5-dichloro-4-iodopyridine (9.72 g, 53% yield). The filtrate was evaporated and the residue purified by chromatography on silica, eluting with a gradient of 50-100% CH2Cl2 in isohexane. Fractions containing product were combined and evaporated and the residue triturated with MeOH to leave a second crop of 2,5-dichloro-4- iodopyridine (5.74 g, 31% yield); 1H NMR spectrum: (300 MHz, DMSO) δ 7.85 (IH, s), 8.34 (IH, s).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2009/153589, 2009, A1 Location in patent:Page/Page column 117