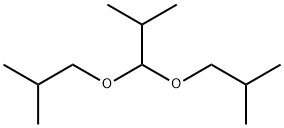
Propane, 2-methyl-1,1-bis(2-methylpropoxy)- synthesis
- Product Name:Propane, 2-methyl-1,1-bis(2-methylpropoxy)-
- CAS Number:13262-24-3
- Molecular formula:C12H26O2
- Molecular Weight:202.33

78-83-1
804 suppliers
$14.00/5mL
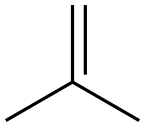
115-11-7
244 suppliers
$45.00/25g

13262-24-3
3 suppliers
inquiry
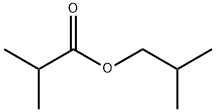
97-85-8
291 suppliers
$21.00/25mL
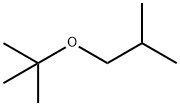
33021-02-2
35 suppliers
$170.00/500mg
Yield:-
Reaction Conditions:
with tetrafluoroboric acid diethyl ether complex;(PCy3)2(CO)RuH(μ-OH)(μ-H)(PCy3)(CO)RuH in decane;chlorobenzene at 100; for 24 h;Schlenk technique;Autoclave;Inert atmosphere;
Steps:
6 Synthesis of 2,5-dimethylhex-2-ene from isobutanol, isobutylene, (PCy3)2(CO)RuH(μ-OH)(μ-H)(PCy3)(CO)RuH and HBF4.Et2O (1:2 molar ratio)
A stock solution consisting of isobutanol (46.8 wt %), n-decane (0.7 wt %) and chlorobenzene (52.5 wt %) was prepared. A portion of the stock solution (6.7071 g stock solution, 42.3 mmoles isobutanol) was transferred to a Schlenk flask followed by 67 μL of HBF4.Et2O (0.5 mmoles). The isobutanol/chlorobenzene/n-decane/HBF4.Et2O mixture was then degassed by 3× freeze/pump/thaw cycles. In the glovebox, the ruthenium compound (PCy3)2(CO)RuH(μ-OH)(μ-H)(PCy3)(CO)RuH (0.2042 g, 0.24 mmoles) was massed into an oven-dried 75 mL Hastelloy C autoclave equipped with a stir bar, followed by the isobutanol solution. The autoclave was then sealed, removed from the glovebox and isobutylene (2.3 g, 41 mmoles) was charged into the autoclave. The autoclave was then place in a 100° C. oil bath and the reaction mixture stirred at this temperature for 24 hours. Afterwards, the autoclave was cooled to room temperature, vented and opened. An aliquot was removed from the reactor, filtered through a plug of SiO2, the SiO2 plug was then flushed with an equal volume of 4% methanol in dichloromethane and the dichloromethane/methanol flush combined with the reaction filtrate was then analyzed by GC using the method listed above. GC analysis revealed that the product 2,5-dimethylhex-2-ene had formed, but was present in small amounts. Confirmation of its existence was confirmed by GC/MS and by spiking the product mixture with known 2,5-dimethylhex-2-ene. Quantification revealed that the product formed in <1% selectivity. The primary products that formed were identified by GC/MS. The primary products were 2-methyl-1,1-bis(2-methylpropoxy)propane, 1-tert-butoxy-2-methylpropane and 2-methylpropyl 2-methylpropanoate and were formed in 46%, 39% and 6% selectivity at 43% isobutanol conversion.
References:
US2015/246940,2015,A1 Location in patent:Paragraph 0052

56-23-5
0 suppliers
$25.00/10ml

78-83-1
804 suppliers
$14.00/5mL

13262-24-3
3 suppliers
inquiry
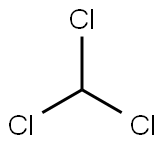
67-66-3
0 suppliers
$33.60/500ml
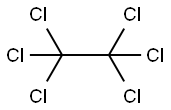
67-72-1
284 suppliers
$11.00/25g
![1-Propene, 3,3'-[(2-methylpropylidene)bis(oxy)]bis[2-methyl-](/CAS/20200611/GIF/6290-52-4.gif)
6290-52-4
0 suppliers
inquiry

13262-24-3
3 suppliers
inquiry
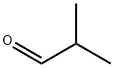
78-84-2
397 suppliers
$10.00/25mL

13262-24-3
3 suppliers
inquiry

97-85-8
291 suppliers
$21.00/25mL

78-83-1
804 suppliers
$14.00/5mL
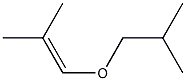
6623-96-7
0 suppliers
inquiry