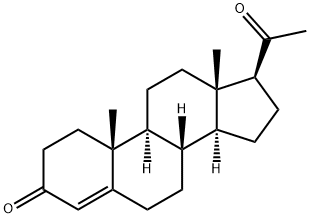
Progesterone synthesis
- Product Name:Progesterone
- CAS Number:57-83-0
- Molecular formula:C21H30O2
- Molecular Weight:314.46

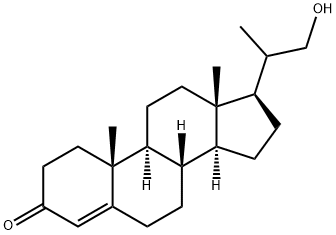
60966-36-1
54 suppliers
inquiry

57-83-0
811 suppliers
$17.00/1g
Yield:57-83-0 99%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;oxygen;acetylacetone copper;N,N-diethylaniline in 1-methyl-pyrrolidin-2-one;N,N-dimethyl-formamide at 0; for 2 h;Inert atmosphere;Solvent;Reagent/catalyst;Temperature;
Steps:
4 Preparation of progesterone (1):
In a dry filled with nitrogen equipped with a thermometer,(2) (16.53 g; Fw: 330.50; 50 mmol) was added in a 250 mL three-necked flask,N, N-dimethylformamide (150 mL)Anhydrous acetylacetone copper (2.62 g; Fw: 261.76; 10.0 mmol)NMP (1.00 g; Fw: 99.13; 10.0 mmol)TEMPO (1.56 g; Fw: 156.25; 10.0 mmol)N, N-diethylaniline (1.49 g; Fw: 149.23; 10.0 mmol). The system temperature was then lowered to 0 ° C and the dry oxygen was introduced. With the oxygen into the system gradually become brown, in the oxygen into the process of continuous exotherm, maintaining the system temperature at 0 . After about 2.0 hours of introduction, after the reaction was completed, 2M hydrochloric acid was slowly added dropwise to the reaction system,pH to neutral, the organic phase was separated and the aqueous phase was extracted with methylene chloride. The organic phases were then combined with pale yellow syrup and treated with anhydrous sulfuric acid Magnesium dry. The solvent was concentrated under reduced pressure and then recrystallized from acetone to give 17.20 g of a white solid, yield: 99.0%.
References:
CN107129516,2017,A Location in patent:Paragraph 0030; 0031; 0032; 0033; 0034; 0035; 0036-0044
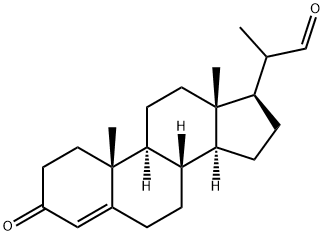
24254-01-1
51 suppliers
inquiry

57-83-0
811 suppliers
$17.00/1g
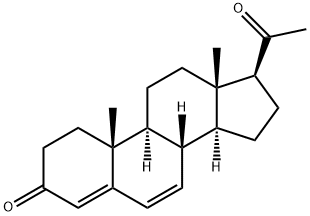
1162-56-7
205 suppliers
$75.00/5G

57-83-0
811 suppliers
$17.00/1g
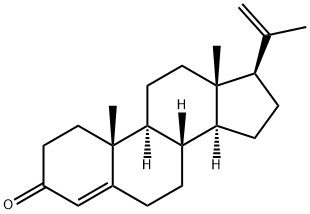
23638-55-3
0 suppliers
inquiry

57-83-0
811 suppliers
$17.00/1g
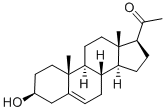
145-13-1
494 suppliers
$28.00/500mg

57-83-0
811 suppliers
$17.00/1g