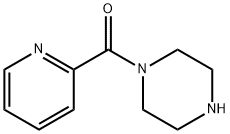
(PIPERAZIN-1-YL)(PYRIDIN-2-YL) METHANONE synthesis
- Product Name:(PIPERAZIN-1-YL)(PYRIDIN-2-YL) METHANONE
- CAS Number:39639-98-0
- Molecular formula:C10H13N3O
- Molecular Weight:191.23
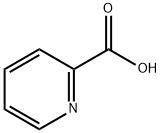
98-98-6
622 suppliers
$5.00/10g
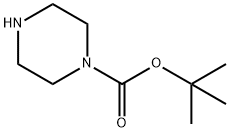
57260-71-6
737 suppliers
$5.00/5g

39639-98-0
37 suppliers
$65.00/50mg
Yield:39639-98-0 59%
Reaction Conditions:
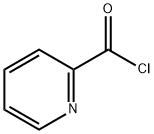
Stage #1: 2-Picolinic acidwith N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride in dichloromethane;N,N-dimethyl-formamide at 20; for 0.5 h;
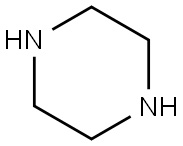
Stage #2: 1-t-Butoxycarbonylpiperazinewith triethylamine in dichloromethane;N,N-dimethyl-formamide at 0 - 20; for 18 h;
Stage #3: with trifluoroacetic acid in dichloromethane at 0 - 20; for 18 h;
Steps:
4.1.2 General procedure for the preparation of intermediates 13-15
General procedure: To a stirred solution of the appropriate carboxylic acid (10-12) (8.0mmol) and HOBt (8.4mmol) in dichloromethane (DCM) (48mL) and DMF (8mL) was added EDC (8.4mmol) at room temperature. The reaction mixture was stirred for another 30min at room temperature (r.t.) and then triethylamine (TEA) (8mmol) and 9 (8mmol) were added. The reaction mixture was then stirred for 18h. After completion of the reaction (monitored by TLC), the mixture was concentrated under reduced pressure, and DCM (300mL) and redistilled water (100mL) were added. The water phase was washed with DCM (2×300mL), and the combined organic phases were washed successively with saturated solutions of NaHCO3 and NaCl. The organic layer was dried over Na2SO4, filtered and concentrated [65]. The crude residue was filtered through a short column of silica gel using DCM/MeOH (50:1 v/v) as eluentTo a solution of the resulting acyl derivative in DCM (20mL) was slowly added TFA (64mmol) at 0°C. The reaction mixture was stirred for an additional 15min at 0°C and then for 18h at r.t. The mixture was diluted with DCM (300mL) and quenched with an ice-cold saturated aqueous solution of Na2CO3 (75mL). The organic layer was then extracted with DCM (3×200mL). The combined organic layers were then dried over Na2SO4, filtered and concentrated under reduced pressure [66]. The crude residue was purified by silica gel chromatography (DCM:MeOH=100:0→90:10 v/v). The 1H NMR spectrum and reaction yield for compound 13 can be found at Supplementary Information
References:
Juza, Radomir;Vojtechova, Iveta;Stefkova-Mazochova, Kristyna;Dehaen, Wim;Petrasek, Tomas;Prchal, Lukas;Kobrlova, Tereza;Janousek, Jiri;Vlcek, Premysl;Mezeiova, Eva;Svozil, Daniel;Karasova, Jana Zdarova;Pejchal, Jaroslav;Stark, Holger;Satala, Grzegorz;Bojarski, Andrzej J.;Kubacka, Monika;Mogilski, Szczepan;Randakova, Alena;Musilek, Kamil;Soukup, Ondrej;Korabecny, Jan [European Journal of Medicinal Chemistry,2022,vol. 232,art. no. 114193]
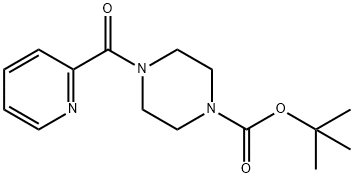
389628-28-8
13 suppliers
inquiry

39639-98-0
37 suppliers
$65.00/50mg

98-98-6
622 suppliers
$5.00/10g

39639-98-0
37 suppliers
$65.00/50mg