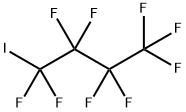
Perfluorobutyl iodide synthesis
- Product Name:Perfluorobutyl iodide
- CAS Number:423-39-2
- Molecular formula:C4F9I
- Molecular Weight:345.93
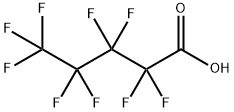
2706-90-3
151 suppliers
$12.00/1g

423-39-2
243 suppliers
$6.00/5g
Yield:423-39-2 336 g
Reaction Conditions:
with iodine in ethanol at 80 - 100; for 13 h;Reagent/catalyst;
Steps:
1.2; 2.2; 3.2; 4.2 Preparation of S2, perfluoroiodobutane
2 kg of the aluminate ionic liquid prepared in the step S1 is added to the reaction vessel equipped with the reflux device,Then, 264 g of perfluoropentanoic acid was added, and the temperature was raised to 80 ° C, and 2.5 mol of iodine in ethanol was slowly added dropwise over 5 hours.Heating was continued to 100 ° C for 8 hours, and the reaction solution was allowed to stand for separation to separate the aluminate ionic liquid layer and the product layer.The product layer was purified by rectification to obtain 336 g of perfluoroiodobutane with a HPLC purity of 98.2%.
References:
CN109651073,2019,A Location in patent:Paragraph 0022; 0025-0027; 0032; 0035-0037; 0042; 0045; 0046
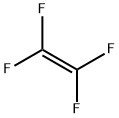
116-14-3
48 suppliers
inquiry
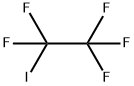
354-64-3
163 suppliers
$45.00/5G
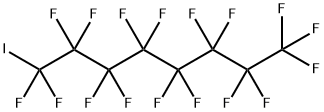
507-63-1
247 suppliers
$5.00/1g

423-39-2
243 suppliers
$6.00/5g
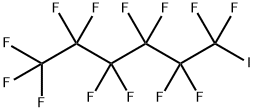
355-43-1
241 suppliers
$10.00/10g
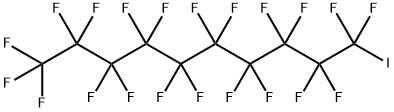
423-62-1
149 suppliers
$10.00/1g

116-14-3
48 suppliers
inquiry

354-64-3
163 suppliers
$45.00/5G

507-63-1
247 suppliers
$5.00/1g

423-39-2
243 suppliers
$6.00/5g

355-43-1
241 suppliers
$10.00/10g

116-14-3
48 suppliers
inquiry

354-64-3
163 suppliers
$45.00/5G

507-63-1
247 suppliers
$5.00/1g
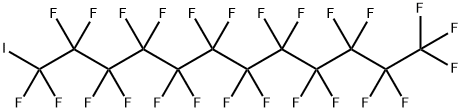
307-60-8
46 suppliers
$32.00/1g

423-39-2
243 suppliers
$6.00/5g

355-43-1
241 suppliers
$10.00/10g

423-62-1
149 suppliers
$10.00/1g
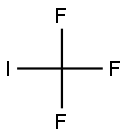
2314-97-8
181 suppliers
$40.00/25g
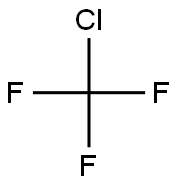
75-72-9
1 suppliers
inquiry
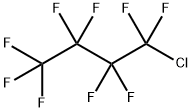
558-89-4
24 suppliers
$180.00/5g

423-39-2
243 suppliers
$6.00/5g