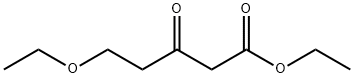
Pentanoic acid, 5-ethoxy-3-oxo-, ethyl ester synthesis
- Product Name:Pentanoic acid, 5-ethoxy-3-oxo-, ethyl ester
- CAS Number:1519-56-8
- Molecular formula:C9H16O4
- Molecular Weight:188.22
Yield:1519-56-8 80%
Reaction Conditions:
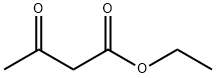
Stage #1: ethyl acetoacetatewith sodium hydride in tetrahydrofuran; for 1 h;
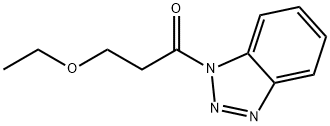
Stage #2: 1-(3-ethoxypropanoyl)-1H-1,2,3-benzotriazole in tetrahydrofuran at 20;
Stage #3: with ammonia;water;ammonium chloride in tetrahydrofuran; for 2 h;Heating / reflux;
Steps:
18.B
Part B A lL round-bottomed flask was charged with sodium hydride (11.7 g of a 60% dispersion in oil, 293 mmol). The sodium hydride was washed with hexanes (2x); then THF (300 mL) was added to the flask. A solution of ethyl acetoacetate (34.6 g, 266 mmol) in THF (100 mL) was then added dropwise via addition funnel under a nitrogen atmosphere. A thick white precipitate formed at this point. After stirring for 1 hour, a solution of l-(3-ethoxypropanoyl)-lH-l,2,3-benzotriazole (58.3 g, 266 mmol) in THF (100 mL) was added via addition funnel. The solution became homogeneous, then eventually a cloudy yellow mixture. This mixture was allowed to stir at room temperature under a nitrogen atmosphere overnight. The following morning, a solution of ammonium chloride (47.0 g, 878 mmol) and ammonium hydroxide (11.2 mL) in de-ionized water (50 mL) was added, and the resultant solution was heated to reflux for 2 hours. The volatile solvents were then removed by rotary evaporation, and the remaining aqueous layer was adjusted to pH 4-5 by addition of aqueous IN HCl. The aqueous layer was then extracted with EtOAc (4 x 200 mL), and the combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated to a yellow oil. This material was purified by suction filter chromatography on silica gel, eluting with 3/1 hexane/EtOAc, to afford 40.2 g (80% yield) of ethyl 5-ethoxy-3-oxopentanoate as a yellow oil. Analysis by 1H NMR revealed material of sufficient purity to carry forward
References:
WO2007/28129,2007,A1 Location in patent:Page/Page column 73
4324-38-3
50 suppliers
$12.07/Price $/1gm:
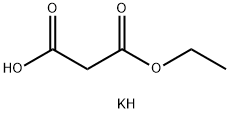
6148-64-7
392 suppliers
$5.00/10g

1519-56-8
2 suppliers
inquiry
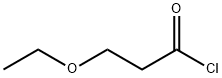
49775-37-3
11 suppliers
inquiry

1519-56-8
2 suppliers
inquiry

4324-38-3
50 suppliers
$12.07/Price $/1gm:

1519-56-8
2 suppliers
inquiry