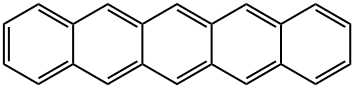
Pentacene synthesis
- Product Name:Pentacene
- CAS Number:135-48-8
- Molecular formula:C22H14
- Molecular Weight:278.35
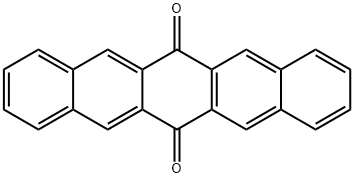
3029-32-1
115 suppliers
$60.00/100mg

135-48-8
192 suppliers
$29.00/25mg
Yield:135-48-8 54%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran for 0.5 h;Inert atmosphere;Cooling with ice;
Steps:
16
Phthalaldehyde (4.02g, 30mmol) and 1,4-cyclohexanedione (1.68g, 15.0mmol) were dissolved in absolute ethanol, stirred evenly and added with NaOH aqueous solution (15mL, 15%), the reaction system immediately changed Into tan.The reactant was stirred at 60°C for 4 hours. After the reaction was completed, it was cooled to room temperature. The solid was filtered and washed with acetone until the filtrate was colorless to obtain 3.95 g of a yellow solid product of 6,13-pentacenequinone with a yield of 85%. Grind the product into powder for later use.Under the protection of nitrogen, add 6,13-pentacenequinone (2.0g, 6.5mmol) and dry tetrahydrofuran 100mL to the dried vial, and quickly add LiAlH4(0.98g, 25mmol)under ice bath conditionsThe solid powder is gradually returned to room temperature under stirring and the suspension is heated to reflux for 30 min.Then the system was cooled to room temperature, and then the hydrochloric acid solution was slowly added under the ice-salt bath. After the addition was completed, the system was heated and refluxed for 3 hours after no gas was generated.After the reaction, the precipitate was filtered, and the filter cake was washed with deionized water (2×30mL), dichloromethane (2×30mL), methanol (2×30mL) and ether (2×30mL) successively, and the remaining after drying The solid repeats the above steps again, and finally a dark blue solid powder of pentacene is obtained with a yield of 54%.In the pressure bottle, add 27.8g pentacene (0.1mol), 43g vinylene carbonate (0.5mol), and react at 220°C for 24h.Cool to room temperature and methanol precipitation to obtain the addition product.The above product was dissolved in 5 g of potassium hydroxide in ethanol, and reacted under reflux for 8 hours, washed and dried to obtain diol.The above product was dissolved in a mixture of 200 mL of dichloromethane and 8 mL of dimethyl sulfoxide, 12 mL of trifluoroacetic anhydride was added dropwise at -78°C, 25 mL of triethylamine was added dropwise after the reaction for 2 hours, and the reaction was continued for 2 hours with stirring. It was dried with sodium sulfate and recrystallized to obtain 22.0 g of product with a yield of 66%.
References:
Liaoning Aoke Chemical Co., Ltd.;Jiangsu Aoke Chemical Co., Ltd.;Zhu Jianmin;Gao Haiyang;Dong Zhenpeng;Liu Zhaobin;Gao Jie;Shi Junsheng CN111377827, 2020, A Location in patent:Paragraph 0155-0158

904913-36-6
2 suppliers
inquiry

135-48-8
192 suppliers
$29.00/25mg
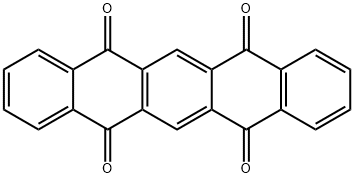
23912-79-0
40 suppliers
$52.00/5g
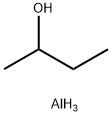
2269-22-9
207 suppliers
$10.00/5g

135-48-8
192 suppliers
$29.00/25mg
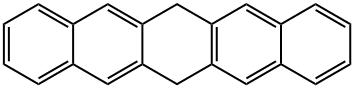
13579-08-3
2 suppliers
inquiry

135-48-8
192 suppliers
$29.00/25mg