
octadecyl (9Z,12Z)-octadeca-9,12-dienoate synthesis
- Product Name:octadecyl (9Z,12Z)-octadeca-9,12-dienoate
- CAS Number:17673-53-9
- Molecular formula:C36H68O2
- Molecular Weight:532.92

67-56-1
751 suppliers
$9.00/25ml

1190-63-2
43 suppliers
$150.00/500mg

17673-53-9
7 suppliers
inquiry
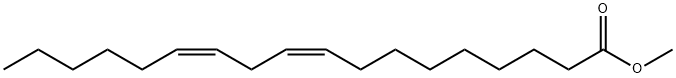
112-63-0
259 suppliers
$15.00/5g
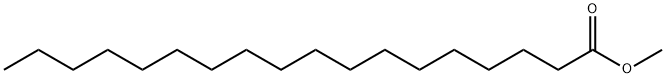
112-61-8
410 suppliers
$8.00/25g
Yield:-
Reaction Conditions:
Stage #1: hexadecyl stearate;stearyl linoleatewith potassium hydroxide;water in methanol at 55; for 1.5 h;
Stage #2: methanolwith sulfuric acid in water at 20 - 55; for 1.5 h;Product distribution / selectivity;
Steps:
1; 7
Samples were uniformly distributed by grinding for 10 -15 sec in a room-temperature coffee bean grinder. Samples could be processed in the state obtained, e.g., wet, dry, freeze- dried, or semi-frozen. Samples (0.5g wet, dry, or semi-frozen sample), (0.25g freeze-dried sample), or oils (20 μl) were placed into a 16 x 125 mm screw-cap Pyrex culture tube to which 1.0 ml Cl 3:0 internal standard (0.5mg C13:0/ml methanol), 0.7 ml 10 N KOH in water, and 5.3 ml methanol was added. The tube was incubated in a 55 °C water bath for 1.5 h with vigorous 5 sec hand-shaking every 20 min to properly permeate, dissolve and hydrolyze the sample. After cooling below room temperature in a cold tap water bath, 0.58 ml of 24 N H2SO4 in water was added. (Care is taken in the preparation of the stock solutions IO N KOH and 24 N H2SO4, especially as the H2SO4 solution is extremely exothermic.) The tube was mixed by inversion, and with precipitated K2SO4 present, was incubated again in a 55 °C water bath for 1.5 h with 5 sec hand-shaking every 20 min. After FAME synthesis, the tube was cooled in a cold tap water bath. Three ml of hexane was added and the tube was vortex-mixed for 5 min on a multi-tube vortex. The tube was centrifuged for 5 min in a tabletop centrifuge and the hexane layer, containing the FAME, was placed into a gas chromatography (GC) vial. The vial was capped and placed at -20 0C until GC analysis; Direct FAME Synthesis Applied to Wax Esters, Cholesteryl Derivatives, and Fatty Acid Salts The ideal FAME synthesis method would be able to analyze fatty acids from samples of wax esters, cholesteryl lipid derivatives, and alkyl methane sulfonates (Palmquist and Jenkins, 2003). We applied the direct FAME synthesis method to such families of fatty acids and the results are shown in Table 6. Direct FAME synthesis was able to identify the fatty acids in wax esters as represented by palmityl stearate (saturated series) and stearyl linoleate (unsaturated series), cholesteryl lipid derivatives as represented by cholesteryl oleate, and fatty acid salts, as represented by oleyl methane sulfonate. As the second component in each of these compounds was a fatty alcohol, and not a fatty acid, it was not converted to the FAME. This is as it should be, since we were analyzing strictly for fatty acid components. Depending on the concentration of these very hydrophobic families of fatty acids, full quantification might require more shaking and a longer incubation time during the first step of direct FAME synthesis.
References:
WO2008/86495,2008,A1 Location in patent:Page/Page column 18; 23; 30