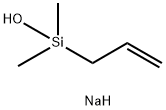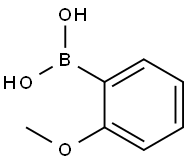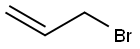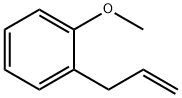
O-ALLYLANISOLE synthesis
- Product Name:O-ALLYLANISOLE
- CAS Number:3698-28-0
- Molecular formula:C10H12O
- Molecular Weight:148.2
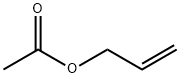
591-87-7
187 suppliers
$10.00/10g
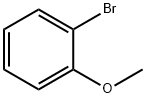
578-57-4
298 suppliers
$6.00/10g

3698-28-0
27 suppliers
$121.00/5g
Yield:3698-28-0 95%
Reaction Conditions:
Stage #1: 2-bromoanisolewith magnesium;lithium chloride in tetrahydrofuran at 20;Inert atmosphere;
Stage #2: with iron(III)-acetylacetonate in tetrahydrofuran at 0; for 0.0833333 h;
Stage #3: Allyl acetate in tetrahydrofuran; for 2 h;
Steps:
1 Coupling of Allyl Acetate with 2-methoxyphenylmagnesium Bromide
Example 1
Coupling of Allyl Acetate with 2-methoxyphenylmagnesium Bromide
Under protective gas, 63 mg of magnesium turnings, 126 mg of anhydrous lithium chloride, 4 ml of dry tetrahydrofuran and 2.4 mmol of 2-bromoanisole were reacted at room temperature to give the Grignard compound.Then the dark-colored solution formed was cooled to 0° C. and a solution of 35.3 mg of iron(III) acetylacetonate (5 mol %) in 2 ml of dry tetrahydrofuran was added and the mixture was stirred for five minutes. Then 2 mmol of allyl acetate were added dropwise and the reaction mixture was stirred for 2 h. For workup, hydrolysis was effected with 5 ml of saturated sodium hydrogencarbonate solution and the mixture was extracted three times with 10 ml each time of ethyl acetate. The combined organic phases were dried over magnesium sulfate, concentrated and purified by column chromatography on silica gel (eluent: cyclohexane-ethyl acetate). 95% of theory of 2-allylanisole was isolated.
References:
US2013/184485,2013,A1 Location in patent:Paragraph 0062; 0063
77-78-1
301 suppliers
$22.00/25g
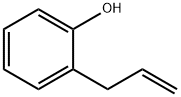
1745-81-9
267 suppliers
$14.00/5g

3698-28-0
27 suppliers
$121.00/5g

1745-81-9
267 suppliers
$14.00/5g

74-88-4
346 suppliers
$15.00/10g

3698-28-0
27 suppliers
$121.00/5g