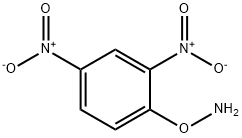
O-(2,4-dinitrophenyl)hydroxylamine synthesis
- Product Name:O-(2,4-dinitrophenyl)hydroxylamine
- CAS Number:17508-17-7
- Molecular formula:C6H5N3O5
- Molecular Weight:199.12
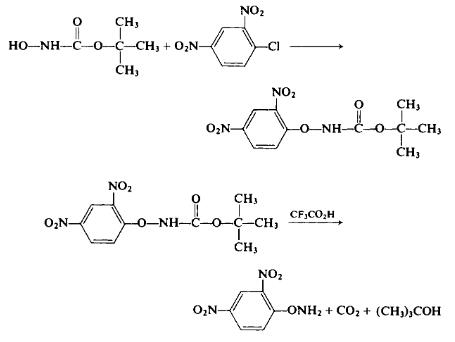
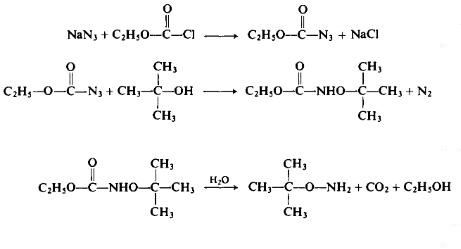
Recently it was discovered that the alkylation of ethyl JV-hydroxy-carbamate under alkaline conditions, particularly in a DMF medium at 60°C in the presence of sodium bicarbonate, leads to the ultimate formation of O-alkylated hydroxylamines. On the other hand, at 80-85°C, the direct alkylation without the presence of a base ultimately leads to N-alkylhydrox-ylamines (see Table I) [59]. The reaction of ethylazidoformate with an alcohol, while perhaps hazardous, may have some merit (Eqs. 31-33). The overall yield, based on ethyl chloroformate, is said to be on the order of 60%.
60506-35-6
24 suppliers
$65.00/500mg

17508-17-7
212 suppliers
$5.00/250mg
Yield:17508-17-7 98%
Reaction Conditions:
with hydrazine hydrate in dichloromethane at 0; for 4 h;
Steps:
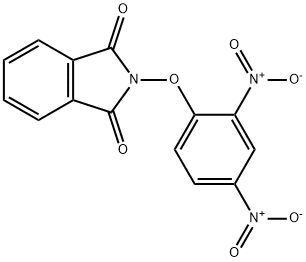
Preparation of Compound 1-2
Preparation of Compound 1-2 2- (2,4-nitrophenoxy) isoindoline-1,3-dione 17.74g (53.88m) and then hydrazine mono-hydrate in yujihan state to 0 dissolved in dichloromethane 350 7.6 (157.33m after the addition of ) was stirred for 4 hours. When the reaction was completed, the reaction mixture poured into a 1N HCl at 0 stirred and the resulting solid was then filter. Filter the solids are washed with MeCN and the filtrate extracted with MC and the organic layer to obtain the desired compound 1-2 10.50g (98%) and dried over MgSO4
References:
Heesung Material Co., Ltd;Jeong, Sujin;Kim, Gi -Yong;Hong, Jang Mi;Yum, Song Jin;Lee, Ju Dong;Jong, Jong-Han;Kim, Min jin;Noh, Young-Seok KR101551895, 2015, B1 Location in patent:Paragraph 0171-0172; 0180-0181
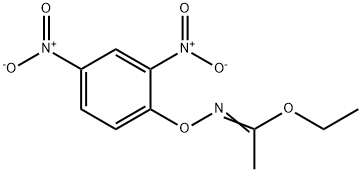
54322-32-6
41 suppliers
$48.20/1g

17508-17-7
212 suppliers
$5.00/250mg
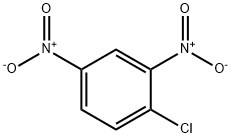
97-00-7
11 suppliers
$17.67/10gm:

17508-17-7
212 suppliers
$5.00/250mg

60506-35-6
24 suppliers
$65.00/500mg
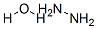
7803-57-8
4 suppliers
$20.10/50g

17508-17-7
212 suppliers
$5.00/250mg

54322-32-6
41 suppliers
$48.20/1g

17508-17-7
212 suppliers
$5.00/250mg