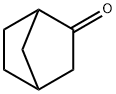
NORCAMPHOR synthesis
- Product Name:NORCAMPHOR
- CAS Number:497-38-1
- Molecular formula:C7H10O
- Molecular Weight:110.15
![bicyclo[2.2.1]heptan-4-ol](/CAS/20180601/GIF/51566-98-4.gif)
51566-98-4
8 suppliers
inquiry

497-38-1
169 suppliers
$13.00/1g
Yield:-
Reaction Conditions:
with pyridinium chlorochromate;molecular sieve in dichloromethane;toluene
Steps:
R.1 Reference Example 1
Then, 2.8 g (0.025 mole) of the obtained (-)-(1S,2S,4R)-exo-norborneol was dissolved in 56 ml of methylene chloride, and 8.1 g (1.5 mole) of pyridinium chlorochromate (PCC) and 1 g of molecular sieve 4A were added thereto. The reaction was conducted at a temperature of 25° C. to 30° C. for 1 hour. Then, the reaction mixture was diluted with 56 ml of toluene, and the resultant was allowed to pass through a column of 28 g silica gel to remove insoluble materials. The elude was concentrated and dried to give crude (+)-norcamphor as a white crystal line powder.
References:
Shionogi & Co., Ltd. US5686285, 1997, A
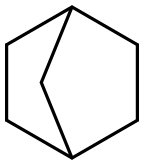
279-23-2
52 suppliers
$90.00/1g

497-38-1
169 suppliers
$13.00/1g
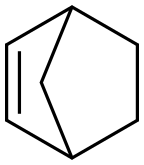
498-66-8
222 suppliers
$10.00/5g

497-38-1
169 suppliers
$13.00/1g
![Bicyclo[2.2.1]heptane, 2-methoxy-, (1R,2R,4S)-rel-](/CAS/20210305/GIF/10395-53-6.gif)
10395-53-6
0 suppliers
inquiry

497-38-1
169 suppliers
$13.00/1g
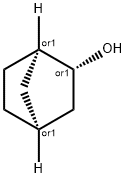
497-37-0
66 suppliers
$33.89/5g

497-38-1
169 suppliers
$13.00/1g