Nandrolone synthesis
- Product Name:Nandrolone
- CAS Number:434-22-0
- Molecular formula:C18H26O2
- Molecular Weight:274.4
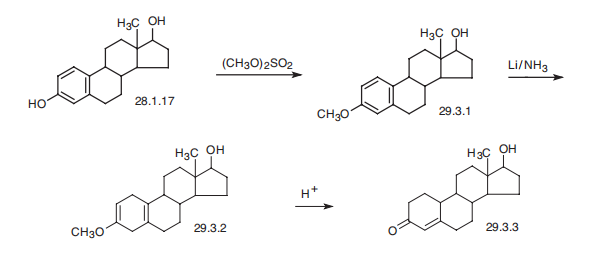
734-32-7
223 suppliers
$300.00/1g

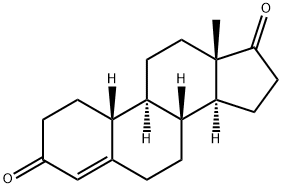
434-22-0
253 suppliers
$33.00/1mg
Yield:434-22-0 53%
Reaction Conditions:
with zygowilliopsis sp. WY7905 in aq. phosphate buffer at 30; pH=8; for 24 h;Enzymatic reaction;stereoselective reaction;
Steps:
2.9. Transformation of C-18, C-19 and C-21 steroids by the resting cells
General procedure: C-18, C-19 and C-21 steroids (0.75 g/l, 1.5 mg), such as estrone(3), 9-dehydroandrostenedione (7), norandrostenedione (9), estra-4,9-diene-3,17-dione (11), 19-hydroxyandrost-4-ene-3,17-dione(13), etc. separately dissolved in Tween 80 (1% w/v) were addedinto 2 ml of reaction mixtures containing glucose (10 g/l, 10 mg)and resting cells (0.2 g wet weight) resuspended in phosphate buffer(100 mM, pH 8.0). The mixture was shaken at 30 C for 24 h,then worked-up and analyzed as described in Section 2.5.
References:
Liu, Yuanyuan;Wang, Yu;Chen, Xi;Wu, Qiaqing;Wang, Min;Zhu, Dunming;Ma, Yanhe [Steroids,2017,vol. 118,p. 17 - 24]
1091-93-6
16 suppliers
inquiry

434-22-0
253 suppliers
$33.00/1mg
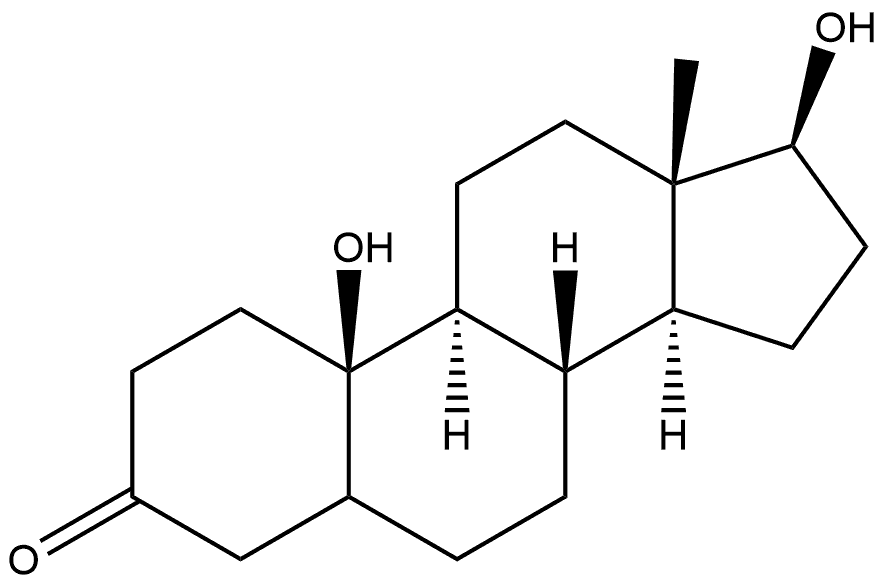
86846-56-2
0 suppliers
inquiry

434-22-0
253 suppliers
$33.00/1mg
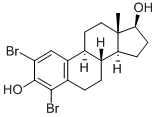
19590-55-7
12 suppliers
inquiry

434-22-0
253 suppliers
$33.00/1mg
