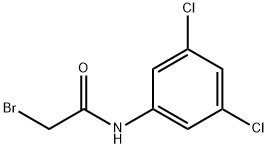
N1-(3,5-DICHLOROPHENYL)-2-BROMOACETAMIDE synthesis
- Product Name:N1-(3,5-DICHLOROPHENYL)-2-BROMOACETAMIDE
- CAS Number:57339-11-4
- Molecular formula:C8H6BrCl2NO
- Molecular Weight:282.95
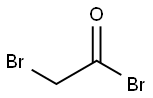
598-21-0
310 suppliers
$10.00/10g
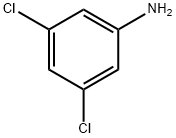
626-43-7
323 suppliers
$10.00/1g

57339-11-4
25 suppliers
$72.50/250mg
Yield:57339-11-4 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 1.25 h;Inert atmosphere;
Steps:
4.6.1. General procedure F: formation of the N-arylbromoacetamides 21a-i
General procedure: To a solution of substituted aniline 22a-i (0.81 mmol) and triethylamine (0.89 mmol) in DCM (2 mL) at 0 °C was added bromoacetyl bromide (0.81 mmol) dropwise over 15 min, and stirring was continued for an additional 1 h at 0 °C. The mixture was dilutedwith DCM (10 mL), washed with HCl (2 10 mL), H2O (1 10 mL),sat. aqueous NaHCO3 (1 10 mL), brine (1 10 mL), dried (Na2SO4),and the solvent removed in vacuo to give bromoacetamides 21a-i.
References:
Hung, Joy M.;Arabshahi, Homayon J.;Leung, Euphemia;Reynisson, Jóhannes;Barker, David [European Journal of Medicinal Chemistry,2014,vol. 86,p. 420 - 437]
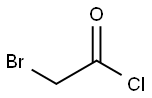
22118-09-8
172 suppliers
$21.21/10gm:

626-43-7
323 suppliers
$10.00/1g

57339-11-4
25 suppliers
$72.50/250mg