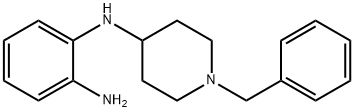
N1-(1-BENZYL-4-PIPERIDYL)BENZENE-1,2-DIAMINE synthesis
- Product Name:N1-(1-BENZYL-4-PIPERIDYL)BENZENE-1,2-DIAMINE
- CAS Number:57718-47-5
- Molecular formula:C18H23N3
- Molecular Weight:281.4
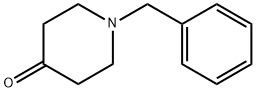
3612-20-2
488 suppliers
$6.00/10g
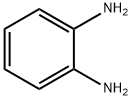
95-54-5
516 suppliers
$9.00/1g

57718-47-5
25 suppliers
$40.00/250mg
Yield:-
Reaction Conditions:
with sodium tris(acetoxy)borohydride;silica gel in tetrahydrofuran;methanol;ethyl acetate;
Steps:
95.A Step A
Step A 1-N-(1-Benzyl-piperidin-4-yl)-benzene-1,2-diamine To a solution of 1.0 g 1-benzyl4-piperidone and 1.14 g of 1,2-phenylenediamine in 15 mL of THF was added two spatula tips of molecular sieves 4?. After stirring at room temperature for 10 minutes, 1.34 g of sodium triacetoxyborohydride was added and the reaction was stirred at room temperature for 18 hours. The reaction was quenched with 50 mL of CH3OH and diluted with EtOAc. After filtering molecular sieves, the filtrate was concentrated under reduced pressure. The residue was partitioned between 50 mL of EtOAc and 30 ml saturated NaHCO3 aqueous solution. After separating layers, the aqueous phase was extracted with 2*50 mL EtOAc. The combined organic phases were washed with 15 mL of brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography eluding with 100% EtOAc, then 90% EtOAc in CH3OH to give 666 mg of the title compound as a solid. 1H NMR (400 MHz, CDCl3): δ1.56 (m, 2H), 2.07 (m, 2H), 2.18(m, 2H), 2.87 (d, J=11.6 Hz, 2H), 3.29(m, 1H), 3.56(s, 2H), 6.69 (m, 3H), 6.72(m, 1H), 7.25-7.61(m, 5H). ESI-MS 282 (M+H); HPLC A: 1.92 min.
References:
US6248755,2001,B1
7255-89-2
2 suppliers
inquiry

57718-47-5
25 suppliers
$40.00/250mg
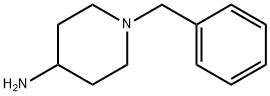
50541-93-0
368 suppliers
$6.00/5g

57718-47-5
25 suppliers
$40.00/250mg
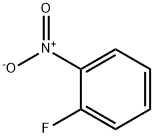
1493-27-2
511 suppliers
$5.00/10g

57718-47-5
25 suppliers
$40.00/250mg