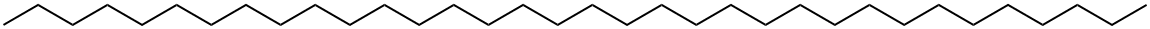
N-TETRATRIACONTANE synthesis
- Product Name:N-TETRATRIACONTANE
- CAS Number:14167-59-0
- Molecular formula:C34H70
- Molecular Weight:478.92
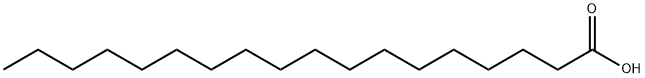
57-11-4
1274 suppliers
$6.00/100g
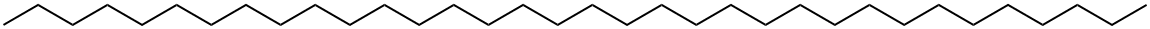
14167-59-0
122 suppliers
$49.79/250mg
Yield:14167-59-0 37.5%
Reaction Conditions:
with sodium methylate in methanol at 40 - 60;Product distribution / selectivity;Electrolysis;
Steps:
1; 3
Example 1Electrocoupling of Long-chain Fatty AcidsAbout 3.0 to about 4.0 mmol of fatty acid was dissolved in methanol, followed by neutralization with about 0.3 to about 0.6 mL of 1M sodium methoxide. A potentiostat/galvanostat with a 100-V maximum compliance voltage (Princeton Applied Research Model 173) maintained a constant current between the platinum electrodes (1.5 cm×1.5 cm and 2.5 cm×1.0 cm, spaced <0.5 cm apart). The electrolysis was carried out in a water-jacketed cell to maintain a constant temperature, which is generally set at a temperature between about 40° C. and about 60° C. A magnetic stir bar was used to agitate the reaction mixture. When an electrical charge equivalent to 1.3 Faradays per mole of the starting acid at the specified current density (generally between 0.05 and 0.12 A cm-2, or about 0.18-0.63 A) passed through the reaction mixture, the electrolysis was halted. The reaction mixture was acidified with a few drops of concentrated HCl, the addition of which converted methoxide to methanol and protonated carboxylate ions. Following evaporation of methanol, the crude product was dissolved in 50 mL of hexanes, transferred to a 125 mL separatory funnel, and washed with three 75 mL volumes of water at 60° C.Table 1 shows the coupling product obtained from corresponding fatty acids.; Example 3Synthesis of n-tetratriacontane (12)Compound 12 was synthesized according to the method of Example 1. Table 3 summarizes the yield and current efficiency for the conversion of stearic acid (4) to n-tetratriacontante. The yields are lower than those obtained for the preparation of (11) above. The maximum yield (50%) was obtained under the following conditions: at 60° C. and 0.45 A cm-2, and 40° C. and 0.23 A cm-2.
References:
Archer-Daniels-Midland Company US7582777, 2009, B2 Location in patent:Page/Page column 18-19

57-11-4
1274 suppliers
$6.00/100g

629-78-7
200 suppliers
$15.00/25g

6765-39-5
72 suppliers
$85.00/1g
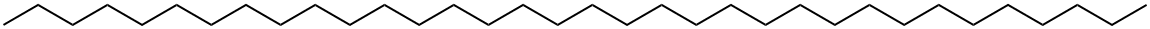
14167-59-0
122 suppliers
$49.79/250mg
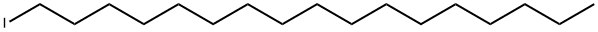
26825-83-2
0 suppliers
inquiry
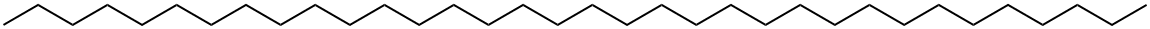
14167-59-0
122 suppliers
$49.79/250mg
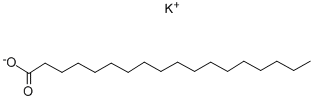
593-29-3
278 suppliers
$68.00/25g
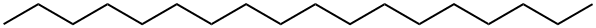
593-45-3
267 suppliers
$17.00/25g
14167-59-0
122 suppliers
$49.79/250mg

62154-85-2
4 suppliers
inquiry
14167-59-0
122 suppliers
$49.79/250mg