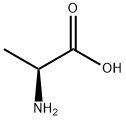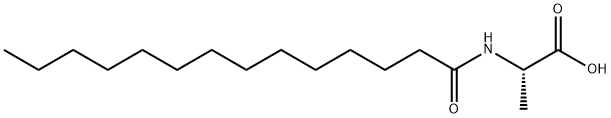
N-Tetradecanoyl-L-alanine synthesis
- Product Name:N-Tetradecanoyl-L-alanine
- CAS Number:71448-29-8
- Molecular formula:C17H33NO3
- Molecular Weight:299.45
Yield: 27%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 20; for 5.25 h;
Steps:
40.1 Step 1: tetradecanoyl-L-alanine.
To a solution of L-alanine (1.6 g, 17.96 mmol), sodium bicarbonate (3.02 g, 35.9 mmol) in THF (40.0 mL)/ Water (40mL) at 20 °C was added tetradecanoyl chloride (2.216 g, 8.98 mmol) dropwise over 15 min. The reaction mixture was stirred at r.t. for 5 h. LCMS indicated completion of reaction. The reaction mixture was diluted with water (30 ml), and extracted with EtOAc (50 ml *2 ).The aqueous phase was acidified with 1 M hydrochloric acid. The resulting solid was filtered through a Buchner funnel, rinsed with water (40 mL), and collected to give the crude tetradecanoyl-L-alanine as a white solid (800 mg, 27%). LCMS (ESI) m/z calcd for C17H33NO3:299. Found: 300 (M+H)+.1HNMR (400 MHz, DMSO-d6) d8.02-7.91 (m, 1H), 4.16-4.05 (m, 1H), 2.05 (t, J = 7.2 Hz, 2H), 1.48-1.41 (m, 2H), 1.26-1.17 (m, 23H), 0.83 (t, J = 6.8 Hz, 3H). Step 2: ((2R,3S,5R)-2-ethynyl-5-(2-fluoro-6-(((4-methoxyphenyl)diphenylmethyl)amino)- 9H-purin-9-yl)-3-((4-methoxyphenyl)diphenylmethoxy)tetrahydrofuran-2-yl)methyl- tetradecanoyl-L-alaninate. Tetradecanoyl-L-alanine (1 g, 3.34 mmol) was dissolved in DCM (30 mL) and added EDC (3.20 g, 16.70 mmol) and DMAP (2.040 g, 16.70 mmol). The resulting mixture was stirred for 40 min at room temperature. Then, ((2R,3S,5R)-2- ethynyl-5-(2-fluoro-6-(((4-methoxyphen-yl)diphenylmethyl)amino)-9H-purin-9-yl)-3-((4- methoxyphenyl)diphenylmeth-oxy)tetrahydro-furan-2-yl)methanol (1.399 g, 1.670 mmol) was added and the resulting mixture was stirred overnight at room temperature. LCMS indicated completion of reaction. The reaction mixture was concentrated to dryness under vacuum to afford the crude product. The crude product was pre-absorbed on silica and purified on a silica column (120 g) using a 0%-60% pet. ether-EtOAc solvent gradient over 60 mins, Flow rate: 70 mL/min. The appropriate fractions were identified by UV absorbance (254 nm), combined and evaporated under vacuum to give the title product as a white solid (800 mg, 19%). LCMS (ESI) m/z calcd for C69H75FN6O7:1119, Found:1120(M+H)+.1HNMR (400 MHz, CDCl3) d7.55-7.49 (m, 4H), 7.42-7.37 (m, 2H), 7.33-7.18 (m, 18H), 6.80 (t, J = 12 Hz, 4H) 5.90 (dd, J = 24.4, 7.6 Hz, 1H), 4.47-4.28 (m, 2H), 4.15- 4.06 (m, 4H), 3.78 (s, 3H),3.74(d, J = 8.0 Hz, 1H), 2.82 (d, J = 8.0 Hz, 1H), 2.05 (s, 4H), 1.29-1.24(m, 22H), 1.19-1.13 (m, 2H), 1.11-1.05 (m, 2H), 0.85 (t, J = 6.4 Hz, 3H). Step 3: ((2R,3S,5R)-5-(6-amino-2-fluoro-9H-purin-9-yl)-2-ethynyl-3-hydroxytetrahydro- furan-2-yl)methyl tetradecanoyl-L-alaninate. To a stirred solution of ((2R,3S,5R)-2-ethynyl- 5-(2-fluoro-6-(((4-methoxyphenyl)diphenylmethyl) amino)-9H-purin-9-yl)-3-((4- methoxyphenyl)di-phenylmethoxy)tetrahydrofuran-2-yl)methyl tetradecanoyl-L-alaninate (800 mg, 0.715 mmol) in DCM (3 mL) at 15 °C was added TFA (300μL, 3.89 mmol) dropwise. The reaction mixture was stirred at 15 °C for 1 h. LCMS indicated completion of reaction. The reaction mixture was diluted with NaHCO3 (30 ml), and extracted with DCM (15 ml * 2). The combined organic phases were washed with brine (15 ml). The organic layers were dried over anhydrous sodium sulphate and concentrated under reduced pressure to afford crude product as a white solid. The sample was pre-absorbed on silica and purified on a silica column (80 g) using a 0%-10% DCM-MeOH solvent gradient over 30 mins, Flow rate: 45 mL/min. The appropriate fractions were identified by UV absorbance (254 nm), combined and evaporated in vacuo to give ((2R,3S,5R)-5-(6-amino- 2-fluoro-9H-purin-9-yl)-2-ethynyl-3-hydroxytetrahydro-furan-2-yl)methyl tetradecanoyl-L- alaninate (189 mg, 46%) as a white solid. LCMS (ESI) m/z calcd for C29H43FN6O5:574. Found: 575 (M+H)+.1HNMR (400MHz, CDCl3) d 7.95 (s, 1H), 6.32 (dd, J = 7.2, 4.8 Hz, 1H), 6.05 - 6.07 ( m, 3H), 4.75( t, J = 6.4 Hz, 1H), 4.61 (d, J = 11.6 Hz, 1H), 4.52( t, J = 6.8 Hz, 1H), 4.42 (d, J = 11.6, 1H), 3.31 (s, 1H), 2.98-3.02 (m, 1H), 2.79 (s, 1H), 2.63-2.70 (m, 1H), 2.21 ( t, J = 7.6 Hz, 2H), 1.58 - 1.63 (m, 2H), 1.40(d, J= 7.2 Hz,3H) 1.24-1.31 ( m, 20H) , 0.83 - 0.89 ( m, 3H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;VIIV HEALTHCARE COMPANY;DE LA ROSA, Martha Alicia;MILLER, John F.;TEMELKOFF, David;VELTHUISEN, Emile Johann;NAIDU, B. Narasimhulu;SAMANO, Vicente WO2020/178767, 2020, A1 Location in patent:Page/Page column 90-91
112-64-1
201 suppliers
$29.12/25G

71448-29-8
41 suppliers
inquiry
338-69-2
596 suppliers
$5.00/10g

2425-54-9
251 suppliers
$26.00/25mL

71448-29-8
41 suppliers
inquiry