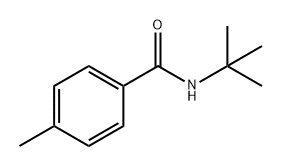
N-(tert-butyl)-4-methylbenzamide synthesis
- Product Name:N-(tert-butyl)-4-methylbenzamide
- CAS Number:42498-32-8
- Molecular formula:C12H17NO
- Molecular Weight:191.27
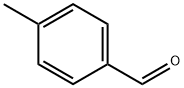
104-87-0
507 suppliers
$6.00/25g
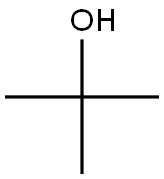
75-65-0
753 suppliers
$10.00/10ml

42498-32-8
15 suppliers
$80.00/100mg
Yield:42498-32-8 98%
Reaction Conditions:
Stage #1: 4-methyl-benzaldehydewith sodium azide;trifluorormethanesulfonic acid;acetic acid at 40; for 2 h;Schmidt Reaction;
Stage #2: tert-butyl alcoholwith trifluorormethanesulfonic acid at 40; for 2 h;Ritter Amidation;
Steps:
General experimental procedure for the synthesis Amides 4
General procedure: To a magnetically stirred solution of benzaldehyde (1 mmol) and NaN3 (1.5 mmol) in AcOH (0.2 mL), TfOH (2 mmol) was added and the reaction mixture was heated at 40 °C for 1-2 h. On the complete conversion of aldehyde (monitored by TLC), alcohol (1 mmol) was added along with TfOH (1 mmol). The reaction was continued to heating at 90 °C (in the case of t-butanol the reaction temperature was 40 °C) for another 3-5 h. After the completion, the reaction was cooled to 0 °C and saturated aq NaHCO3 was added slowly. The precipitate formed was filtered and washed with plenty of cold water and dried to obtain the desired secondary benzamide as white solid in 98% yield. (Compounds obtained were pure hence, no further purification or recrystallisation was required).
References:
Singh, Garima;Dada, Ravikrishna;Yaragorla, Srinivasarao [Tetrahedron Letters,2016,vol. 57,# 39,p. 4424 - 4427] Location in patent:supporting information
540-88-5
366 suppliers
$24.00/25mL
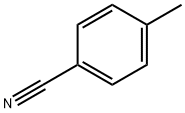
104-85-8
324 suppliers
$11.00/25g

42498-32-8
15 suppliers
$80.00/100mg

104-85-8
324 suppliers
$11.00/25g

75-65-0
753 suppliers
$10.00/10ml

42498-32-8
15 suppliers
$80.00/100mg
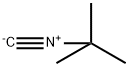
7188-38-7
167 suppliers
$22.39/250MG
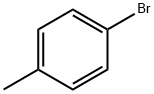
106-38-7
401 suppliers
$14.00/5g

42498-32-8
15 suppliers
$80.00/100mg
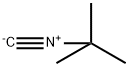
7188-38-7
167 suppliers
$22.39/250MG
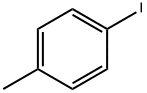
624-31-7
431 suppliers
$9.00/25g

42498-32-8
15 suppliers
$80.00/100mg