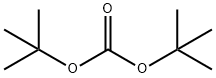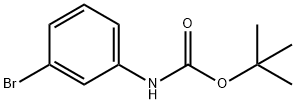
N-(TERT-BUTOXYCARBONYL)-3-BROMOANILINE synthesis
- Product Name:N-(TERT-BUTOXYCARBONYL)-3-BROMOANILINE
- CAS Number:25216-74-4
- Molecular formula:C11H14BrNO2
- Molecular Weight:272.14

24424-99-5
833 suppliers
$13.50/25G
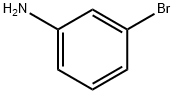
591-19-5
322 suppliers
$10.00/10g

25216-74-4
55 suppliers
$17.00/1g
Yield: 100%
Reaction Conditions:
in sodium hydroxide
Steps:
14.1 Step 1.
Step 1. N-t-Boc-3-bromoaniline 3-bromoaniline (10 g, 58.1 mmol) and di-tert-butyldicarbonate (19.0 g, 87.1 mmol) were dissolved in 2M aqueous sodium hydroxide and heated at reflux for 1 hour. After cooling to ambient temperature, the reaction mixture was extracted with ethyl acetate. The organic layer was washed (saturated aqueous ammonium chloride, 1*; water, 1*; and brine, 2*), dried (MgSO4), filtered, concentrated in vacuo, and dried under high vacuum to provide N-t-Boc-3 bromoaniline as a colorless solid (15.8 g, 100%). mp 83° C. 1 H NMR (300 MHz, CDCl3) δ 7.66 (1H, br m), 7.08-7.23 (3H, m), 6.46 (1H, br s), 1.52 (9H, s). MS m/e 272/274 (M+H)+, 289/291 (M+NH4)+.
References:
Abbott Laboratories US5407959, 1995, A

24424-99-5
833 suppliers
$13.50/25G

591-19-5
322 suppliers
$10.00/10g
105946-57-4
1 suppliers
inquiry

25216-74-4
55 suppliers
$17.00/1g

24424-99-5
833 suppliers
$13.50/25G

591-19-5
322 suppliers
$10.00/10g
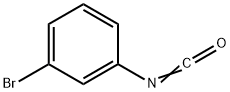
23138-55-8
77 suppliers
$22.69/1g

25216-74-4
55 suppliers
$17.00/1g
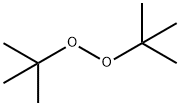
110-05-4
271 suppliers
$22.00/100mL
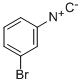
148854-09-5
5 suppliers
inquiry

25216-74-4
55 suppliers
$17.00/1g