N,N'-Diphenylbenzidine synthesis
- Product Name:N,N'-Diphenylbenzidine
- CAS Number:531-91-9
- Molecular formula:C24H20N2
- Molecular Weight:336.43
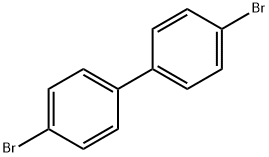
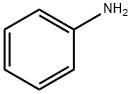
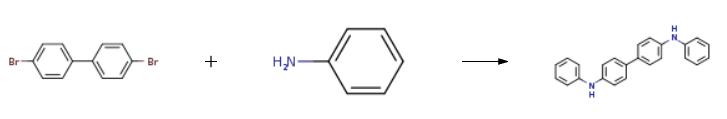
3.12 g (10mmol) of 4,4'-dibromodiphenyl, 2.3 mL (25 mmol) of aniline, 2.9 g (30 mmol) of t-BuONa, 183 mg (0.2 mmol) of Pd2(dba)3, and 20 mg (0.1 mmol) of P(t-Bu)3 were dissolved in 30 mL of toluene, and then stirred at 90°C for 3 hrs. The reaction mixture was cooled to room temperature and three times extracted with distilled water and diethyl ether. Precipitates in an organic layer were filtered, washed with acetone and diethyl ether, and then dried in vacuum to obtain 0.3g of the intermediate compound C (yield: 90%).
Yield:531-91-9 90%
Reaction Conditions:
with sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0);tri-tert-butyl phosphine in toluene at 90; for 3 h;
Steps:
2
3.12 g (10mmol) of 4,4'-dibromodiphenyl, 2.3 mL (25 mmol) of aniline, 2.9 g (30 mmol) of t-BuONa, 183 mg (0.2 mmol) of Pd2(dba)3, and 20 mg (0.1 mmol) of P(t-Bu)3 were dissolved in 30 mL of toluene, and then stirred at 90°C for 3 hrs. The reaction mixture was cooled to room temperature and three times extracted with distilled water and diethyl ether. Precipitates in an organic layer were filtered, washed with acetone and diethyl ether, and then dried in vacuum to obtain 0.3g of the intermediate compound C (yield: 90%). The structure of the intermediate compound C was identified with 1H NMR. 1H NMR (DMSO-d6, 400MHz) δ (ppm) 8.22 (s, 2H), 7.48 (d,4H), 7.23 (t, 4H), 7.10 (dd, 8H), 6.82 (t, 2H); 13C NMR (DMSO-d6, 100MHz) δ (ppm) 145.7, 144.3, 133.7, 131.4, 128.7, 121.2, 119.2, 118.9.
References:
EP1661888,2006,A1 Location in patent:Page/Page column 11-12
108-90-7
656 suppliers
$10.00/25G
92-87-5
186 suppliers
$31.00/250mg

531-91-9
346 suppliers
$7.00/1g
122-39-4
341 suppliers
$14.00/5g

531-91-9
346 suppliers
$7.00/1g