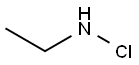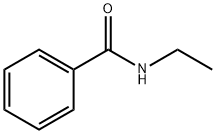
N-ETHYLBENZAMIDE synthesis
- Product Name:N-ETHYLBENZAMIDE
- CAS Number:614-17-5
- Molecular formula:C9H11NO
- Molecular Weight:149.19
Yield:614-17-5 241 mg
Reaction Conditions:
with tert.-butylhydroperoxide;tetra-(n-butyl)ammonium iodide in water;acetonitrile at 80; for 0.416667 h;Flow reactor;Green chemistry;
Steps:
4.2. General procedure
General procedure: 5 mmol of amine (1) was dissolved in 50 mL CH3CN, which was placed into syringe A. And 5 mmol of N-Chlorosuccinimide (NCS) was dissolved in 50 mL CH3CN, which was placed into syringe B. Methylarene (2, 25 mmol, 10 eq.), tert-butylammonium iodide (TBAI, 2.5 mmol,1 eq.) and TBHP (70 wt% in water,12.5 mmol, 5 eq.) were dissolves in 50 mL CH3CN/H2O (CH3CN:H2O 4:1), which was placed into syringe C. The flow rate of syringes A, B and C were 0.1 mL/min, 0.1 mL/min, and 0.2 mL/min, respectively. And the temperature of the two oil baths was set in 50 °C and 80 °C, respectively. The reaction liquid was collected, and then quenched by NaHSO3 solution and extracted with ethyl acetate, washed with H2O. The organic layer was dried over anhydrous sodium sulfate and solvent was removed under vacuum. And the crude product was purified by flash chromatography on silica gel by gradient elution with ethyl acetate in petroleum ether to obtain the product
References:
Fang, Zheng;Guo, Kai;He, Wei;Liu, Chengkou;Shi, Tingting;Yang, Yuhang;Yang, Zhao;Zhang, Zhimin [Tetrahedron,2020,vol. 76,# 13] Location in patent:supporting information
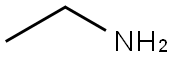
75-04-7
393 suppliers
$10.00/20mg
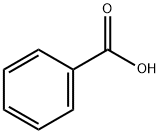
65-85-0
1365 suppliers
$10.00/25g

614-17-5
32 suppliers
$172.00/50mg
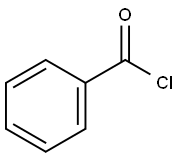
98-88-4
579 suppliers
$10.00/5g

75-04-7
393 suppliers
$10.00/20mg

614-17-5
32 suppliers
$172.00/50mg
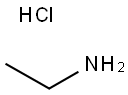
557-66-4
267 suppliers
$26.00/25g
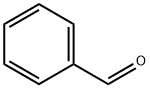
100-52-7
949 suppliers
$20.37/250g

614-17-5
32 suppliers
$172.00/50mg