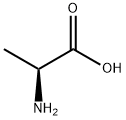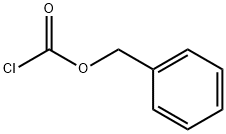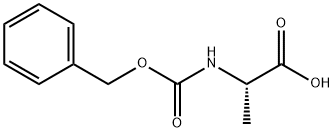
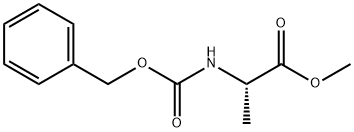
N-Carbobenzyloxy-L-alanine synthesis
- Product Name:N-Carbobenzyloxy-L-alanine
- CAS Number:1142-20-7
- Molecular formula:C11H13NO4
- Molecular Weight:223.23
28819-05-8
92 suppliers
$6.00/250mg

1142-20-7
459 suppliers
$5.00/1g
Yield:1142-20-7 98%
Reaction Conditions:
Stage #1:(S)-2-benzyloxycarbonylamino-propionic acid methyl ester with sodium hydroxide in ethanol;water at 20; for 0.666667 h;
Stage #2: with hydrogenchloride in ethanol;water; pH=2
Steps:
2
5 g (21 mmol) of compound 3 was dissolved in 50 ml of ethanol, 30 ml of 2.5 mM NaOH was added. The reaction was stirred at room temperature for 40 minutes, acidified with 6N HCl to about pH 2. The combined extracts were washed twice with saturated NaCl solution, dried (Na2SO4). The solvent was removed to give 4.59 g (98%) of the desired product as white solid or clear oil. 1H NMR (500 MHz, CDCl3): δ 7.34-7.27 (m, 5H), 5.39 (br, 1H), 5.11 (s, 2H), 4.38 (q, J=7.2 Hz, 1H), 1.43 (d, J=7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 177.78, 155.98, 136.16, 128.68, 128.39, 128.28, 67.27, 49.59, 18.49.
References:
Development Center for Biotechnology US2006/287500, 2006, A1 Location in patent:Page/Page column 2; 3
![L-Alanine, N-[(phenylmethoxy)carbonyl]-, 2-phenylhydrazide](/CAS/20210305/GIF/28861-55-4.gif)
28861-55-4
0 suppliers
inquiry

1142-20-7
459 suppliers
$5.00/1g
![L-Alanine, N-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/50300-96-4.gif)
50300-96-4
0 suppliers
inquiry

1142-20-7
459 suppliers
$5.00/1g
![L-Alanine, N-[(phenylmethoxy)carbonyl]-, 2-amino-2-oxoethyl ester](/CAS/20210305/GIF/4816-87-9.gif)
4816-87-9
0 suppliers
inquiry
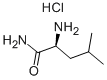
10466-61-2
210 suppliers
$9.00/1g

1142-20-7
459 suppliers
$5.00/1g
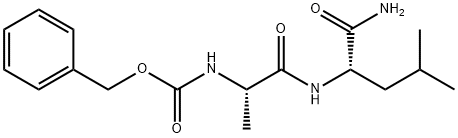
18323-56-3
10 suppliers
inquiry