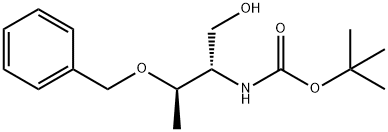
N-BOC-(2S,3S)-2-AMINO-3-BENZYLOXY-1-BUTANOL synthesis
- Product Name:N-BOC-(2S,3S)-2-AMINO-3-BENZYLOXY-1-BUTANOL
- CAS Number:79069-63-9
- Molecular formula:C16H25NO4
- Molecular Weight:295.37
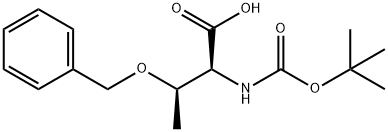
15260-10-3
269 suppliers
$8.00/5g

79069-63-9
12 suppliers
inquiry
Yield:79069-63-9 84%
Reaction Conditions:
Stage #1: N-Boc-O-benzyl-L-threoninewith 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;N-ethyl-N,N-diisopropylamine in ethyl acetate at 0; for 0.166667 h;
Stage #2: with sodium tetrahydroborate in water;ethyl acetate at 0; for 0.583333 h;
Steps:
General procedure for the reduction of carboxylic acids to alcohols using T3P-NaBH4:
General procedure: To a solution of carboxylic acid (10 mmol) in THF (10 mL), DIPEA (11 mmol, 1.42 mL) and 50% T3P in EtOAc (20 mmol, 6.36 mL) were added at 0 °C and the solution was stirred for about 10 min. Then aqueous solution of NaBH4 (10 mmol, 388 mg in 0.3 mL of H2O) was added to the reaction mixture at the same temperature and the reaction was allowed to stir till the completion of the reaction as indicated by TLC. After the completion of the reaction, the solvent was evaporated and the crude alcohol was extracted into EtOAc and the organic phase was washed with 5% citric acid (10 mL × 2), 5% Na2CO3 (10 mL × 2), water, and brine solution. The product was isolated after the evaporation of solvent under reduced pressure and dried over anhydrous Na2SO4.
References:
Nagendra;Madhu;Vishwanatha;Sureshbabu, Vommina V. [Tetrahedron Letters,2012,vol. 53,# 38,p. 5059 - 5063] Location in patent:experimental part
![L-Threonine, N-[(1,1-dimethylethoxy)carbonyl]-O-(phenylmethyl)-, anhydride with 2-methylpropyl hydrogen carbonate (9CI)](/CAS/20211123/GIF/84890-92-6.gif)
84890-92-6
0 suppliers
inquiry

79069-63-9
12 suppliers
inquiry
![Carbamic acid, N-[(1S,2R)-1-(1H-benzotriazol-1-ylcarbonyl)-2-(phenylmethoxy)propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1072841-04-3.gif)
1072841-04-3
0 suppliers
inquiry

79069-63-9
12 suppliers
inquiry
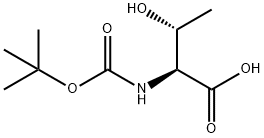
2592-18-9
321 suppliers
$5.00/1g

79069-63-9
12 suppliers
inquiry