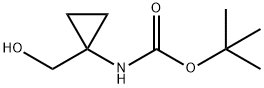
tert-Butyl (1-(hydroxymethyl)cyclopropyl)carbamate synthesis
- Product Name:tert-Butyl (1-(hydroxymethyl)cyclopropyl)carbamate
- CAS Number:107017-73-2
- Molecular formula:C9H17NO3
- Molecular Weight:187.24

24424-99-5
833 suppliers
$13.50/25G
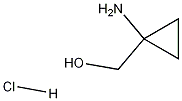
115652-52-3
153 suppliers
$8.00/100mg

107017-73-2
194 suppliers
$8.00/100mg
Yield:107017-73-2 100%
Reaction Conditions:
Stage #1: 1-amino-1-(hydroxymethyl)cyclopropane hydrochloridewith triethylamine in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: di-tert-butyl dicarbonate in tetrahydrofuran at 0 - 20;
Steps:
20.1 Step 1tert-butyl N-[1-(hydroxymethyl)cyclopropyl]carbamate (20B)
Triethylamine (4.93 g,48.76 mmol) was added dropwise to a stirred solution of (1- aminocyclopropyl)methanol hydrochloride(20A) (2 g,16.25 mmol) in THF (50 mL) at 0°C. After 10 min of stirring, di-tert-butyl dicarbonate (7.09 g,32.50mmol) in THF (5 mL) was added dropwise to the mixture at 0°C. The mixture was stirred overnight in room temperature, the solvent was removed in vacuo. The residue was diluted with ethyl acetate (60 mL), washed with water (2×60 mL) and brine (50 mL), dried with Na2SO4 and concentrated. then the title compound (20B) (3g.100%) was obtained as white solid, which was used in the next step without further purification.1H NMR (400 MHz, CDCl3) d 5.05 (s, 1H), 3.59 (s, 2H), 2.40 (s, 1H), 1.44 (s, 9H), 0.83 (m, 4H).
References:
WO2020/185755,2020,A1 Location in patent:Paragraph 00392
88950-64-5
267 suppliers
$9.00/250mg

107017-73-2
194 suppliers
$8.00/100mg
107259-05-2
62 suppliers
$60.00/100mg

107017-73-2
194 suppliers
$8.00/100mg
66494-26-6
53 suppliers
$41.00/250mg

107017-73-2
194 suppliers
$8.00/100mg
![Carbamic acid, [1-[(phenylmethoxy)methyl]cyclopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/308266-24-2.gif)
308266-24-2
0 suppliers
inquiry

107017-73-2
194 suppliers
$8.00/100mg