
N-(3-FLUOROPHENYL)-3-AMINOPROPIONIC ACID synthesis
- Product Name:N-(3-FLUOROPHENYL)-3-AMINOPROPIONIC ACID
- CAS Number:885275-89-8
- Molecular formula:C9H10FNO2
- Molecular Weight:183.18
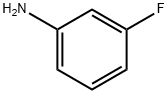
372-19-0
323 suppliers
$10.00/1g
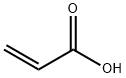
79-10-7
701 suppliers
$20.50/500ml

885275-89-8
35 suppliers
$48.00/250mg
Yield:-
Reaction Conditions:
in water at 20;Reflux;
Steps:
3
To 3-fluoroaniline (5.0 g), water (50 ml) and acrylic acid (3.6 g) were added and heated under reflux for 3 hours. After cooling to room temperature, the reaction mixture was stirred for 3 days and further heated under reflux for 7 hours. After cooling at room temperature, the solvent was distilled off under reduced pressure. To the resulting residue, 8M aqueous sodium hydroxide and chloroform were added to separate the aqueous layer. The separated aqueous layer was adjusted to pH 3 with 12M aqueous hydrochloric acid under ice cooling, and then extracted with chloroform. The extracted organic layer was dried over anhydrous magnesium sulfate and then filtered to remove the desiccant, followed by distilling off the solvent under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluting solvent: n-hexane:ethyl acetate = 4:1) to give 3-[N-(3-fluorophenyl)]aminopropionic acid (4.2 g) as a light-yellow powder. The compound thus obtained (3.8 g) was added to polyphosphoric acid (35 g) heated at 130°C, followed by stirring for 1.5 hours. The reaction mixture was neutralized by addition of water and 8M aqueous sodium hydroxide under ice cooling, and then extracted with chloroform. The extracted organic layer was dried over anhydrous magnesium sulfate and then filtered to remove the desiccant, followed by distilling off the solvent under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluting solvent: n-hexane:ethyl acetate = 9:1 to 4:1 to 2:1) to give the titled compound of low polarity, i.e., 7-fluoro-2,3-dihydro-1H-quinolin-4-one (810 mg) as a yellow powder. On the other hand, a yellow oil obtained as a compound of high polarity was crystallized from a mixture of n-hexane and diethyl ether to give the other titled compound, i.e., 5-fluoro-2,3-dihydro-1H-quinolin-4-one (110 mg) as a yellow powder. 7-Fluoro-2,3-dihydro-1H-quinolin-4-one 1H NMR (300 MHz, CHLOROFORM-D) δ 2.69 (t, J=7.0 Hz, 2 H), 3.59 (td, J=7.0 Hz, 2.2 Hz, 2 H), 4.51 (brs, 1 H), 6.33 (dd, J=10.4, 2.3 Hz, 1 H), 6.44 (td like, J=8.6, 2.3 Hz, 1 H), 7.86 (dd, J=8.9, 6.5 Hz, 1 H). 5-Fluoro-2,3-dihydro-1H-quinolin-4-one 1H NMR (300 MHz, CHLOROFORM-D) δ 2.70 (t, J=6.9 Hz, 2 H), 3.51-3.65 (m, 2 H), 4.56 (brs, 1 H), 6.31-6.48 (m, 2 H), 7.10-7.30 (m, 1 H).
References:
EP2172453,2010,A1 Location in patent:Page/Page column 9

372-19-0
323 suppliers
$10.00/1g

885275-89-8
35 suppliers
$48.00/250mg