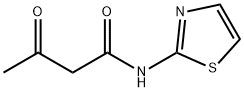
N-(2-THIAZOLYL)ACETOACETAMIDE synthesis
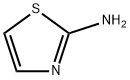
- Product Name:N-(2-THIAZOLYL)ACETOACETAMIDE
- CAS Number:705-87-3
- Molecular formula:C7H8N2O2S
- Molecular Weight:184.22
Yield:705-87-3 89%
Reaction Conditions:
in 5,5-dimethyl-1,3-cyclohexadiene;Reflux;
Steps:
1 General procedure for the synthesis of N-(aryl)-3-oxobutanamides (3a-e)
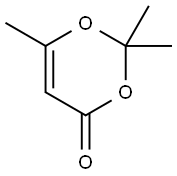
General procedure: N-(Aryl)-3-oxobutanamides (3a-e) were synthesized accordingto the modified method of Clemens via condensation of 2,2,6-trimethyl-1,3-dioxine-4-one with the appropriate arylamine.50The results are represented in Table 7 4.2.1.1
N-(2-Thiazolyl) 3-oxo-butanamide (3a)
Pale yellow crystals; Yield 89%; 1H NMR (DMSO-d6) δ (ppm) 11.32 (s, 2H, NH-amide), 8.93 (s, 1H, NH-DHP), 7.46 (d, J = 4 Hz, 1H, CH-furyl), 6.71 (t, J = 3.6 Hz, 1H, CH-furyl), 6.40 (s, 2H, CH3-thiazole), 5.90 (d, J = 3.2 Hz, 1H, CH-furyl), 5.34 (s, 1H, C4H-DHP), 2.29 (s, 6H, CH3-thiazole), 2.25 (s, 6H, CH3-DHP); IR (KBr) ν (cm-1): 3344.4 (N-H, DHP), 2924.0 (C-H, aliphatic), 1667.6 (C=O, amide), 1598.3 (C=C, alkene); MS m/z (%): 455(35) [M+], 410(12), 399(50), 313(75), 228(79), 202(100), 115(85), 42(21); Anal. Calcd (%) for C7H8N2O2S: C, 45.64; H, 4.38; N, 15.21; S, 17.41. Found: C, 45.78; H, 4.44; N, 15.26; S, 17.34.
References:
Razzaghi-Asl, Nima;Firuzi, Omidreza;Hemmateenejad, Bahram;Javidnia, Katayoun;Edraki, Najmeh;Miri, Ramin [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 22,p. 6893 - 6909]