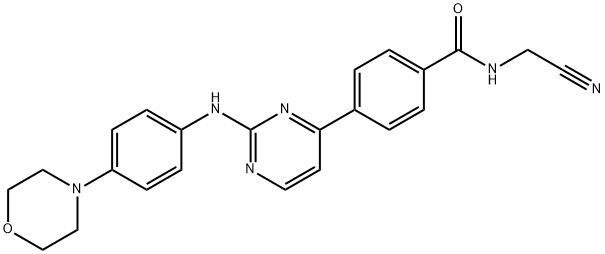
Momelotinib synthesis
- Product Name:Momelotinib
- CAS Number:1056634-68-4
- Molecular formula:C23H22N6O2
- Molecular Weight:414.46
Momelotinib (CYT387) is an ATP-competitive inhibitor of JAK1/JAK2 with IC50 of 11 nM/18 nM, ~10-fold selectivity versus JAK3. Phase 3. It was prepared from 5-1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride with benzotriazol-1-ol by stirring DMF for overnight at room temperature.
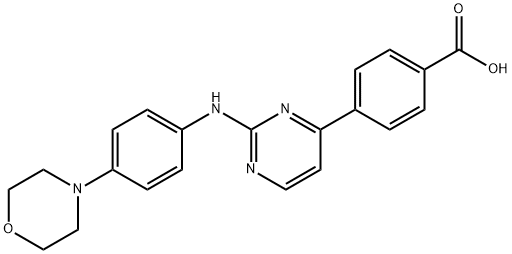
945749-71-3
31 suppliers
inquiry
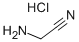
6011-14-9
382 suppliers
$5.00/10g

1056634-68-4
211 suppliers
$61.00/1mg
Yield:1056634-68-4 90%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 20;
Steps:
Synthesis of N-(cyanomethyl)-4-{2-[(4-morpholinophenyl)amino]pyrimidin-4-yl}benzamide (momelotinib)
Et3N (4.1 mL, 30 mmol) was added to a mixture of compound 6(1.88 g, 5.0 mmol) in DMF (20 mL). Then aminoacetonitrile hydrochloride (0.93 g, 10 mmol) was added followed by N-hydroxybenzotriazole (HOBt, 0.81 g, 6.0 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.1 g, 6.0 mmol). The mixture was stirred overnight at room temperature. After removing the solvents under reduced pressure, the residue was dissolved in CH2Cl2 (100 mL) and washed with NaHCO3 aqueous solution. The organic phase was dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by column chromatography on 200-300 mesh silica with EtOAc to afford momelotinib as: Yellow solid; yield 1.86 g (90%); m.p. 232-234 °C (lit.14 238-243 °C); IR: 3365, 3282, 1660, 1596, 1576, 1512, 1456, 1232 cm-1. Anal. calcd for C23H22N6O2: C, 66.65; H, 5.35; N, 20.28; found: C, 66.78; H, 5.49; N, 20.39%. ESI-MS: 415.1 [M + H]+, 437.1 [M + Na]+, 413.2 [M - H]-; 1H NMR (300 MHz, DMSO-d6): δ 9.47 (s, 1H), 9.32 (t, J = 5.4 Hz, 1H), 8.54 (d, J = 5.1 Hz, 1H), 8.27 (d,J = 8.1 Hz, 2H), 8.03 (d, J = 8.1 Hz, 2H), 7.67 (d, J = 8.7 Hz, 2H), 7.40 (d, J = 5.1 Hz, 1H), 6.94 (d, J = 8.7 Hz, 2H), 4.35 (d, J = 5.1 Hz, 2H), 3.74-3.77 (m, 4H), 3.04-3.07 (m, 4H); 13C NMR (75 MHz, DMSO-d6): δ 166.1, 162.4, 160.3, 159.2, 146.2, 139.9, 134.5, 132.8, 127.8, 126.9, 120.3, 117.5, 115.6, 107.6, 66.1, 49.2, 27.7.
References:
Sun, Tong;Xu, Jiaojiao;Ji, Min;Wang, Peng [Journal of Chemical Research,2016,vol. 40,# 8,p. 511 - 513]

945749-71-3
31 suppliers
inquiry

540-61-4
67 suppliers
$65.00/50 mg

1056634-68-4
211 suppliers
$61.00/1mg
2524-67-6
319 suppliers
$8.00/1g

1056634-68-4
211 suppliers
$61.00/1mg
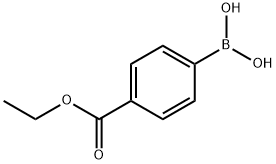
4334-88-7
289 suppliers
$6.00/1g

1056634-68-4
211 suppliers
$61.00/1mg
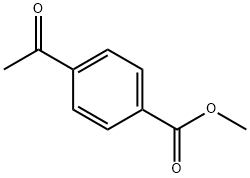
3609-53-8
202 suppliers
$5.00/1g

1056634-68-4
211 suppliers
$61.00/1mg