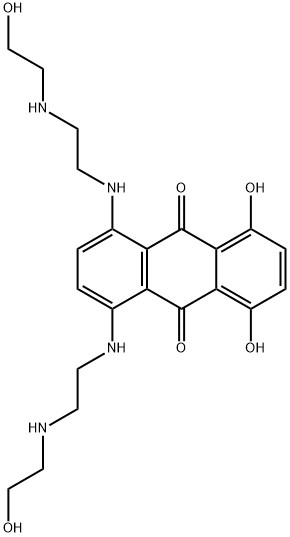
Mitoxantrone synthesis
- Product Name:Mitoxantrone
- CAS Number:65271-80-9
- Molecular formula:C22H28N4O6
- Molecular Weight:444.48
Yield:65271-80-9 38%
Reaction Conditions:
in 1,4-dioxane at 53; for 2.2 h;Inert atmosphere;Temperature;
Steps:
4 Example 4Step 1: Synthesis of APIs:
1 at room temperature, add 10.5g of 1,4,5,8-tetrahydroxyindole leuco in a 250ml three-necked flask, and then evacuate the air in a 250ml three-necked flask with a balloon containing nitrogen or argon;2 After venting the air in a 250 ml three-necked flask, add 59 ml of 1,4 dioxane and start stirring.Until the 1,4,5,8-tetrahydroxyindole leuco is completely dissolved in the 1,4-dioxane;3 After completion of the above 2, 38 g of N-(2-hydroxyethyl)ethylenediamine was added to a constant pressure titration funnel, and then N-(2-hydroxyethyl)ethylenediamine was added dropwise to a 250 ml three-necked flask. Continuous stirring during the dropping process, the dropping time is 14 minutes, and after the end of the dropwise addition, a paste-like viscous liquid is obtained;4 After completing the above 3, a 250 ml three-necked flask containing a paste-like viscous liquid is heated in a water bath, the temperature is controlled at 53 ° C, and the reaction is carried out at this temperature for 2.2 hours. After the reaction, a blue-brown or blue-violet color is obtained. Oily liquid, product D;5 Pour the obtained product D into a stoppered jar, add 170 ml of absolute ethanol, record the liquid scale at this time, and then put the stoppered jar into the water bath to control the heat temperature to 59 °C. And continuously pass dry oxygen dried with anhydrous calcium sulfate into the stoppered jar, and slowly oxidize for 5.5 hours at a temperature of 59 ° C until the color of the reaction liquid changes to bright blue;6 Let the bright blue liquid obtained be cooled to room temperature, and add the remaining amount of ethanol in a stoppered jar until the liquid scale recorded in the above 5 is allowed to stand at room temperature for 30 hours, then the bright blue liquid is poured. Into a Buchner funnel for vacuum filtration to obtain a blue drug substance crystal, that is, product E;Step 2: Refinement of APIs:1 in the beaker, according to the ratio of absolute ethanol to n-hexane volume ratio of 3:1 ratio of 180ml;2 Weigh 9g of product E, pour it into a three-necked bottle, then pour the mixture of the above 1 into a three-necked bottle, and put the three-necked bottle into a water bath to stir and dissolve. The dissolution temperature was controlled to 54 ° C. After complete dissolution, the activated carbon was added to the stoppered three-necked flask, and the mixture was continuously stirred and heated to reflux. The temperature of the hot reflux was controlled to 68 ° C.The control was carried out for 22 minutes. Immediately after the completion of the hot reflux, the filtrate was filtered under reduced pressure to obtain a filtrate. The obtained filtrate was allowed to cool, and crystals were precipitated by ice-cooling overnight, and then subjected to vacuum filtration to obtain a crystal of the drug;3 Configure the mixture in the above 1 again, and then rinse the obtained drug crystals several times until there is no visible reddish-brown blue crystal in the product, which has a clear luster and is evenly granulated. After the rinsing is finished, The crystal is dried in a constant temperature drying oven, the drying temperature is 105 ° C, the drying time is 1.9 hours, and after the drying is finished, it is refined.The medicine, namely mitoxantrone hydrochloride.The content of the obtained mitoxantrone hydrochloride drug was measured by HPLC-UV, and the yield was 38%.
References:
Nanjing Medical University Kangda College;Wang Mingming CN108395379, 2018, A Location in patent:Paragraph 0023; 0025-0077
![5,8-Dihydroxy-1,4-bis-[2-(2-hydroxy-ethylamino)-ethylamino]-2,3-dihydro-anthraquinone](/CAS/20210305/GIF/70476-74-3.gif)
70476-74-3
11 suppliers
inquiry

65271-80-9
191 suppliers
$42.00/50mg
81-59-4
30 suppliers
inquiry

65271-80-9
191 suppliers
$42.00/50mg
131401-54-2
11 suppliers
$109.90/5 mg
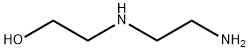
111-41-1
472 suppliers
$6.00/25g

65271-80-9
191 suppliers
$42.00/50mg