
Methyl L-prolinate hydrochloride synthesis
- Product Name:Methyl L-prolinate hydrochloride
- CAS Number:2133-40-6
- Molecular formula:C6H12ClNO2
- Molecular Weight:165.62

67-56-1
752 suppliers
$9.00/25ml
15761-39-4
608 suppliers
$5.00/10g

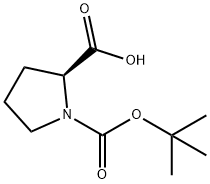
2133-40-6
438 suppliers
$8.00/5g
Yield:2133-40-6 88%
Reaction Conditions:
Stage #1: methanol;1-(tert-butoxycarbonyl)-L-prolinewith thionyl chloride at 70; for 7.5 h;Inert atmosphere;Cooling with ice;
Stage #2: with hydrogenchloride in dichloromethane;ethyl acetate at 20; for 0.0833333 h;Inert atmosphere;
Steps:
20.1 Step 1) : (S) -methyl pyrrolidine-2-carboxylate hydrochloride
To a solution of (S) -1- (tert-butoxycarbonyl) pyrrolidine-2-carboxylic acid (1.50 g, 6.97 mmol) in methanol (20 mL) was added sulfoxide chloride (0.51 mL, 6.97 mmol) dropwise in an ice-bath. The mixture was stirred in the ice-bath for 30 min, and then stirred at 70 for 7 h. The reaction mixture was concentrated, and DCM (8 mL) and a HCl in EtOAc solution (4 M, 6 mL) were added. The resulting mixture was stirred at rt for 5 min, and then concentrated to give the title compound as white thick oil (800 mg, 88) .1H NMR (400 MHz, CD3OD) : δ ppm 4.47 (t, J 7.8 Hz, 1H) , 3.88 (s, 3H) , 3.37-3.45 (m, 2H) , 2.41-2.50 (m, 1H) , 2.07-2.21 (m, 3H) .
References:
WO2016/34134,2016,A1 Location in patent:Paragraph 00324
59936-29-7
224 suppliers
$6.00/5g

2133-40-6
438 suppliers
$8.00/5g
5211-23-4
59 suppliers
$7.00/1g

2133-40-6
438 suppliers
$8.00/5g
147-85-3
996 suppliers
$6.00/25g

2133-40-6
438 suppliers
$8.00/5g