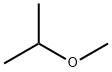
Methyl isopropyl ether synthesis
- Product Name:Methyl isopropyl ether
- CAS Number:598-53-8
- Molecular formula:C4H10O
- Molecular Weight:74.12
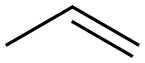
187737-37-7
1 suppliers
inquiry

74-98-6
119 suppliers
$40.00/1mL
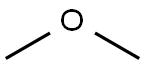
115-10-6
96 suppliers
$75.00/25g

463-49-0
48 suppliers
$125.00/10g
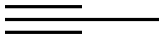
74-99-7
99 suppliers
$45.00/1ml

67-56-1
752 suppliers
$9.00/25ml

598-53-8
28 suppliers
$496.45/5MG
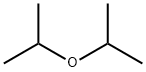
108-20-3
562 suppliers
$25.00/25mL
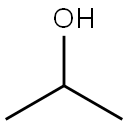
67-63-0
1581 suppliers
$17.00/25ML
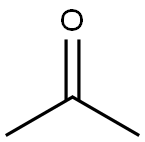
67-64-1
6 suppliers
$19.10/10ml
Yield:-
Reaction Conditions:
with hydrogen;hydrogenation catalyst from SCI of Louisville, Ky., USA at 209; under 16801.7 Torr;
Steps:
6
Example 6Conversion of Oxygenate Impurities in the Presence of Highly Unsaturated Olefin [00271] A feed comprising dimethyl ether, methyl acetylene, and propadiene was exposed to a commercial gas-phase methyl acetylene/propadiene (MAPD) hydrogenation catalyst obtained from SCI of Louisville, Ky., USA. The catalyst was reduced under hydrogen at 232 C. (450 F.) and 360 GHSV and then cooled to reaction temperature under helium flow. Hydrocarbon was fed to the reactor at a rate of 3.2 WHSV with respect to total catalyst weight (960 GHSV, with respect to total catalyst volume). The feed consisted of 84.13 mol % propylene, 9.56 mol % propane, 2.63 mol % methyl acetylene, 3.68 mol % propadiene and 183 ppm dimethyl ether. Hydrogen was co-fed to attain varying H2/MAPD (hydrogen to methyl acetylene and propadiene) molar ratios. [00272] TABLE 2 shows a typical hydrocarbon product distribution at 209 C. and approximately 310 psig. Under these conditions, MAPD conversion was about 87 mol %. In addition, a DME conversion of about 50 mol % was attained. [00273] Major oxygenate products included acetone and methyl isopropyl ether, which could be easily separated from light olefins in a depropanizer distillation column. TABLE 3 shows a product distribution from the converted DME. [TABLE-US-00002] TABLE 2 Reaction Conditions and Product Distribution from MAPD Conversion WRSV (h-1) 3.2 Temperature ( C.) 209 Pressure kPaa (psig) 2240 (309.6) H2/MAPD (mol) 1.10 FEED (mol % except as noted) C3 = 84.13 C3 9.56 Methyl Acetylene 2.63 Propadiene 3.68 DME (ppm) 183 PRODUCT C1 0.00 C2 = 0.05 C2 0.01 C3 11.26 Methyl Acetylene 0.26 Propadiene 0.56 iC4 and nC4 0.03 C4 = 0.03 C4 == 0.04 C5s 0.02 C6s 0.26 Benzene 0.00 C6s + 0.08 DME (ppm) 93 Estimated Oxygenates (ppm) 90 [TABLE-US-00003] TABLE 3 Distribution of Oxygenated Products Oxygenate Product Selectivity (mol %) Methyl Isopropyl Ether 56.7 Acetone 27.7 Isopropanol 7.1 Methanol 5.4 Diisopropyl Ether 3.2
References:
US6717025,2004,B1 Location in patent:Page column 38-39
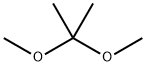
77-76-9
389 suppliers
$9.00/5ml

598-53-8
28 suppliers
$496.45/5MG

67-63-0
1581 suppliers
$17.00/25ML

74-88-4
347 suppliers
$15.00/10g

598-53-8
28 suppliers
$496.45/5MG

67-63-0
1581 suppliers
$17.00/25ML
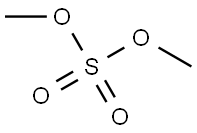
77-78-1
304 suppliers
$22.00/25g

598-53-8
28 suppliers
$496.45/5MG
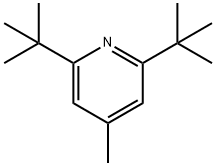
38222-83-2
246 suppliers
$6.00/250mg
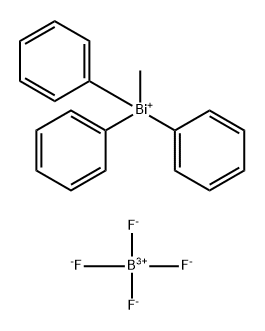
278172-59-1
0 suppliers
inquiry

67-63-0
1581 suppliers
$17.00/25ML

598-53-8
28 suppliers
$496.45/5MG
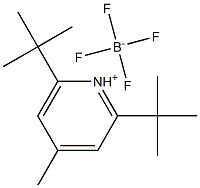
160142-36-9
0 suppliers
inquiry
603-33-8
254 suppliers
$30.00/5g