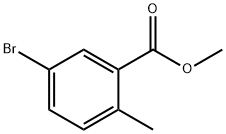
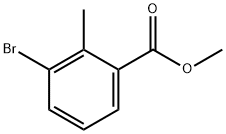
methyl 5-bromo-2-methyl-benzoate synthesis
- Product Name:methyl 5-bromo-2-methyl-benzoate
- CAS Number:79669-50-4
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

67-56-1
751 suppliers
$7.29/5ml-f
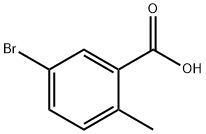
79669-49-1
443 suppliers
$5.00/1g

79669-50-4
192 suppliers
$12.00/5g
Yield:79669-50-4 99%
Reaction Conditions:
Stage #1:methanol;5-bromo-2-methylbenzoic acid with diazomethyl-trimethyl-silane in hexane at 20; for 1.16667 h;
Stage #2: with acetic acid in hexane for 0.75 h;
Steps:
5
Place 5-bromo-2-methyl-benzoic acid (1.0 g, 4.7 mmol) in a 200 mL flask under an N2 atmosphere and add methanol via syringe. Add a 2M solution of diazomethyl- trimethyl-silane in hexane (3.5 mL, 23.0 mmol) drop wise over 10 minutes and stir for 1 hour at room temperature. Add glacial acetic acid (16 mL) and stir for 45 minutes. Dilute with ethyl acetate (100 mL) and wash with 1M aqueous sodium hydroxide solution (30 mL), saturated aqueous sodium bicarbonate solution (30 mL) and brine (30 mL). Dry the organic layer (Na2SO4), filter and concentrate in vacuo to obtain 1.01 g of 5-bromo-2- methyl-benzoic acid methyl ester (99 %). Place 5-bromo-2-methyl-benzoic acid methyl ester (1.04 g, 4.5 mmol) in a 50 mL flask under a N2 atmosphere and add carbon tetrachloride (15 mL). Add N-bromo- succinamide (1.49 g, 8.3 mmol) and 2,2'-azobisisobutyronitrile (40 mg, 0.2 mmol) and fit flask with a condenser and reflux for 4 hours. Cool to room temperature and filter. Concentrate the filtrate and pre-adsorb the crude product onto silica gel. Chromatograph the residue on a Si02 column eluting with dichloromethane in hexane (0 to 50%) to obtain 977 mg of 5-bromo-2-bromomethyl-benzoic acid methyl ester (70%). Using 5-bromo-2-bromomethyl-benzoic acid methyl ester (0. 984 g, 3.20 mmol) and the procedure described in the I st paragraph for the alternative procedure for 5- (4,4, 5, 5-tetramethyl- [1, 3,2] dioxaborolan-2-yl) -2, 3-dihydro-isoindol-1-one, prepare 509 mg of the title compound (75 %).
References:
ELI LILLY AND COMPANY WO2005/73205, 2005, A1 Location in patent:Page/Page column 27-28

79669-49-1
443 suppliers
$5.00/1g

74-88-4
346 suppliers
$15.00/10g

79669-50-4
192 suppliers
$12.00/5g

79669-49-1
443 suppliers
$5.00/1g
18107-18-1
209 suppliers
$33.00/5g

79669-50-4
192 suppliers
$12.00/5g

79669-49-1
443 suppliers
$5.00/1g

79669-50-4
192 suppliers
$12.00/5g
76006-33-2
316 suppliers
$10.00/5g

79669-49-1
443 suppliers
$5.00/1g

74-88-4
346 suppliers
$15.00/10g
99548-54-6
158 suppliers
$5.00/1g

79669-50-4
192 suppliers
$12.00/5g