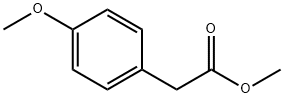
METHYL 4-METHOXYPHENYLACETATE synthesis
- Product Name:METHYL 4-METHOXYPHENYLACETATE
- CAS Number:23786-14-3
- Molecular formula:C10H12O3
- Molecular Weight:180.2

67-56-1
751 suppliers
$7.29/5ml-f
104-01-8
528 suppliers
$6.00/10g

23786-14-3
145 suppliers
$9.00/1g
Yield:23786-14-3 99%
Reaction Conditions:
with thionyl chloride at 0 - 20; for 1.5 h;
Steps:
1.4.4Methyl 2-(4-methoxyphenyl)acetate(17).
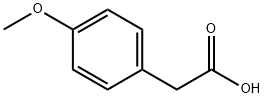
Thionyl chloride (26 ml, 357.60 mmol, 1.64 g/ml) was added dropwise into the anhydrous methanol (100ml) at 0°C. After the addition, p-methoxyphenylaceticacid16(9.90 g, 59.60 mmol) was added, the reaction was transferred to room temperature and reacted for 1h. The reaction solution was extracted with ethyl acetate. The organic phase was washed with saturated sodium chloride, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography to give the compound17(11.91 g, 99% yield) as a white solid.1H NMR (400 MHz, CDCl3) δ 7.20 (d,J= 8.6 Hz, 2H), 6.89-6.83 (m, 2H), 3.79 (s, 3H), 3.68 (s, 3H), 3.57 (s, 2H).HRMS (ESI) (m/z):calcdfor C10H12O3[M+H]+ 181.0865, found. 181.0861.
References:
Li, Guangzhe;Dong, Huijuan;Ma, Yao;Shao, Kun;Li, Yueqing;Wu, Xiaodan;Wang, Shisheng;Shao;Zhao, Weijie [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 16,p. 2327 - 2331] Location in patent:supporting information
104-92-7
427 suppliers
$10.00/10g
38330-80-2
291 suppliers
$14.00/5g

23786-14-3
145 suppliers
$9.00/1g

67-56-1
751 suppliers
$7.29/5ml-f
6343-93-7
68 suppliers
$27.00/1g

23786-14-3
145 suppliers
$9.00/1g
14199-15-6
301 suppliers
$7.00/10g

74-88-4
347 suppliers
$15.00/10g

23786-14-3
145 suppliers
$9.00/1g