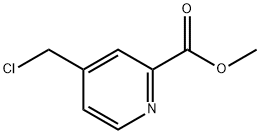
methyl 4-(chloromethyl)pyridine-2-carboxylate synthesis
- Product Name:methyl 4-(chloromethyl)pyridine-2-carboxylate
- CAS Number:1206973-14-9
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61
317335-15-2
86 suppliers
$67.82/250mg

1206973-14-9
25 suppliers
$220.00/100mg
Yield:1206973-14-9 92%
Reaction Conditions:
with methanesulfonyl chloride;triethylamine in dichloromethane; for 24 h;Reflux;
Steps:
4.1.12. Methyl 4-(chloromethyl)picolinate (20)
General procedure: To a stirred solution of methyl 4-(hydroxymethyl)picolinate 16(0.70 g, 4.1 mmol) in dry CH2Cl2 (40 mL) was added Et3N (1.16 mL,8.3 mmol). The mixture was cooled to 0 °C, and mesyl chloride(0.49 mL, 6.3 mmol) was slowly added. The mixture was then heated for 24 h, and quenched at room temperature with a saturated solution of NaHCO3. The aqueous layer was extracted with EtOAc (x3). The organic phase was washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by silica gel chromatography (99/1 CH2Cl2/MeOH) to afford 20 as a brown oil in 92% yield (0.72 g). 1H NMR (300 MHz, CDCl3) δ 8.75(d, J 4.9 Hz, 1H), 8.16 (d, J 1.0 Hz, 1H), 7.52 (dd, J 5.0, 1.7 Hz,1H), 4.61 (s, 2H), 4.03 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.9,149.9, 148.0, 147.3, 125.9, 124.1, 52.7, 43.3. MS (ESI): m/z (%): 188(33), 186 (100) [MH]
References:
Oukoloff, Killian;Coquelle, Nicolas;Bartolini, Manuela;Naldi, Marina;Le Guevel, Rémy;Bach, Stéphane;Josselin, Béatrice;Ruchaud, Sandrine;Catto, Marco;Pisani, Leonardo;Denora, Nunzio;Iacobazzi, Rosa Maria;Silman, Israel;Sussman, Joel L.;Buron, Frédéric;Colletier, Jacques-Philippe;Jean, Ludovic;Routier, Sylvain;Renard, Pierre-Yves [European Journal of Medicinal Chemistry,2019,vol. 168,p. 58 - 77]

67-56-1
751 suppliers
$9.00/25ml
923169-37-3
42 suppliers
$38.00/250mg

1206973-14-9
25 suppliers
$220.00/100mg

923169-37-3
42 suppliers
$38.00/250mg

1206973-14-9
25 suppliers
$220.00/100mg