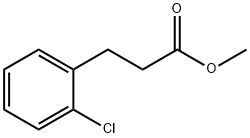
Methyl 3-(2-chlorophenyl)propanoate synthesis
- Product Name:Methyl 3-(2-chlorophenyl)propanoate
- CAS Number:74124-86-0
- Molecular formula:C10H11ClO2
- Molecular Weight:198.65

67-56-1
755 suppliers
$9.00/25ml
1643-28-3
155 suppliers
$8.00/1g

74124-86-0
19 suppliers
$602.00/5g
Yield:74124-86-0 98%
Reaction Conditions:
with thionyl chloride at 20; for 4 h;Reflux;
Steps:
Methyl 3-(2-bromophenyl)propanoate (5a)
To a solution of acid 10a (7.7 g, 33.6 mmol) in methanol (60 mL) stirred at ambient temperature thionyl chloride (0.9 mL, 12 mmol) was added dropwise. The mixture was refluxed for 1 h. Subsequently another thionyl chloride was added in two portions (2 0.9 mL, 24 mmol) under reflux, the second portion was added 1 h after the first one. After next two hours of reflux, the volatile components were distilled off under reduced pressure (70 °C, 0.8 kPa). The light yellow oily residue was dissolved in DCM (60 mL) and washed with saturated aqueous NaHCO3 (60 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to give yellow oily liquid (7.8 g, 96%). Proton NMR data are consistent with literature [10].
References:
Douov, Hana;Hork, Radim;Ruikov, Zdeka;imunek, Petr [Beilstein Journal of Organic Chemistry,2015,vol. 11,p. 884 - 892] Location in patent:supporting information
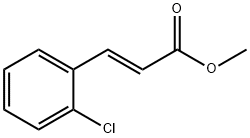
98288-14-3
31 suppliers
$45.00/10mg

74124-86-0
19 suppliers
$602.00/5g
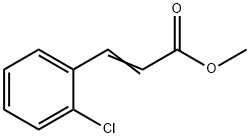
42174-97-0
3 suppliers
inquiry

74124-86-0
19 suppliers
$602.00/5g
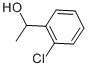
13524-04-4
123 suppliers
$50.79/5g

74124-86-0
19 suppliers
$602.00/5g