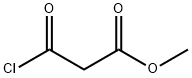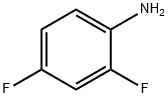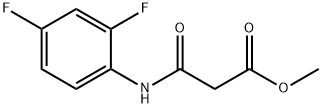
methyl 2-[(2,4-difluorophenyl)carbamoyl]acetate synthesis
- Product Name:methyl 2-[(2,4-difluorophenyl)carbamoyl]acetate
- CAS Number:479690-12-5
- Molecular formula:C10H9F2NO3
- Molecular Weight:229.18
Yield:479690-12-5 68.4%
Reaction Conditions:
with triethylamine at 0 - 20;
Steps:
4
Chlorocarbonylacetic acid methyl ester (1.00 g) was dissolved in tetrahydrofuran (20 ml) under a nitrogen atmosphere, and triethylamine (1.12 ml) and 2,4-difluoroaniline (0.82 ml) were added thereto in an ice water bath, followed by raising the temperature up to room temperature and stirring for 3 hrs and 40 min. Triethylamine (0.56 ml) and 2,4-difluoroaniline (0.39 ml) were added further thereto, followed by stirring at room temperature overnight. Triethylamine (0.25 ml) and 2,4-difluoroaniline (0.17 ml) were added further thereto, followed by stirring at room temperature for 3 hrs. Triethylamine (0.25 ml) and 2,4-difluoroaniline (0.17 ml) were added further thereto, followed by stirring at room temperature for 1 hr and 20 min. The reaction mixture was partitioned between ethyl acetate and 1 N HCl. The organic layer was washed with a saturated aqueous solution of sodium hydrogencarbonate, water and brine in this order, dried over anhydrous sodium sulfate, and concentrated under a reduced pressure. The resultant residue was purified by silica gel column chromatography (Fuji Silysia NH, eluent; hexane:ethyl acetate=1:1). The solvent was evaporated to give a residue, to which diethyl ether-hexane (1:1) was added to suspend. A solid was filtered off and dried under aeration to provide the titled compound (1.14 g, 68.4%) as a pale purple solid. 1H-NMR Spectrum (CDCl3) δ (ppm): 3.53 (2H, s), 3.83 (3H, s), 6.82-6.94 (2H, m), 8.18-8.29 (1H, m), 9.42 (1H, brs).
References:
US2005/277652,2005,A1 Location in patent:Page/Page column 50