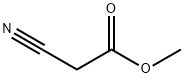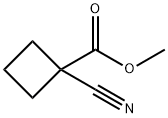
methyl 1-cyanocyclobutanecarboxylate synthesis
- Product Name:methyl 1-cyanocyclobutanecarboxylate
- CAS Number:58920-79-9
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
Yield:58920-79-9 62.81%
Reaction Conditions:
Stage #1: methyl 2-cyanoacetatewith 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethyl-formamide at 10 - 50; for 0.25 h;
Stage #2: 1,3-dibromo-propanewith 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethyl-formamide at -10 - 70; for 0.75 h;
Steps:
1
To methyl cyanoacetate (10 g, 0.1 mol) under N2, N,N-dimethylformamide (DMF, 10 mL) was added at room temperature. After 10 min, the solution was added 1,8-diazabicyclo [5,4,0]undec-7-ene (DBU, 33.4 g, 0.22 mol) at 10-20° C., and then reacted 15 min at 50° C. The mixture was cooled to -5 to -10° C., 1,3-dibromopropane (20.4 g, 0.1 mol) in DMF (10 mL) added by syringe, reacted 15 min at room temperature, rose up to 70° C., and then reacted 30 min. The mixture was evaporated and the residue taken up in H2O and extracted with CHCl3 (3×50 mL). The chloroform phase was evaporated under reduced pressure. The compound 1 was purified by column chromatography (SiO2, hexane/acetone 8:1), yielding 8.74 g (62.81%). MS (ESI): m/z: 140.08 [M+H]+. Anal. Calcd (Found) for C7H9NO2: C, 60.42 (60.74); H, 6.52 (6.58); N, 10.07 (10.21). 1H NMR (D2O, 400 MHz), δ ((ppm): 3.84 (s, 3H, -OCH3), 2.73-2.63 (m, 4H, -C-CH2-CH2-CH2-), 2.17-2.32 (m, 2H, -C-CH2-CH2-CH2-). 13C NMR (D2O, 400 MHz), δ (ppm): 169.17, 128.11, 53.50, 39.38, 31.22, 17.18.
References:
US2007/207087,2007,A1 Location in patent:Page/Page column 4; 7; sheet 1