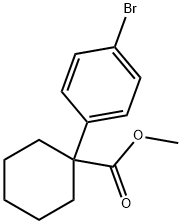
methyl 1-(4-bromophenyl)cyclohexane-1-carboxylate synthesis
- Product Name:methyl 1-(4-bromophenyl)cyclohexane-1-carboxylate
- CAS Number:1236357-63-3
- Molecular formula:C14H17BrO2
- Molecular Weight:297.19
Yield:1236357-63-3 99%
Reaction Conditions:
Stage #1: (4-bromo-phenyl)-acetic acid methyl esterwith sodium hydride in tetrahydrofuran at 35; for 2 h;Inert atmosphere;
Stage #2: 1,5-diiodopentane in tetrahydrofuran at 20 - 35;Inert atmosphere;
Steps:
Preparation of Intermediate methyl 1-(4-bromoDhenyl)cvclohexanecarboxylate (1AE-1); Sodium hydride (12 g, 52 mmol) was suspended in tetrahydrofuran (200 mL) under argon and warmed to 35°C. Methyl 2-(4-bromophenyl)acetate (26mmol) in tetrahydrofuran added drop wise to reaction over 1 hour. The reaction mixture was then kept at this temperature for 1 hour until all gas evolution has ceased. The 1 ,5- diiodopentane (17 g, 52 mmol) was then added drop wise as a solution in tetrahydrofuran (100 mL) and the reaction mixture stirred at 35°C for a further hour and at ambient temperature overnight. After this time, the reaction mixture was cooled to 00C and quenched by the addition of dry silica, filtered and the solvent removed under vacuum. The crude product was then purified by flash chromatography eluting with 33% ethyl acetate in heptane to give methyl 1-(4-bromophenyl)cyclohexanecarboxylate (1AC-1 ) (15.3 g, 99 % yield) as a yellow oil.1 H NMR (400 MHz, CDCI3): 7.45-7.38 (m, 2 H), 7.27-7.24 (m, 2 H), 3.63 (s, 3 H), 2.43 (d, J = 13.3 Hz, 2 H), 1.71-0.80 (m, 8 H) ppm.
References:
WO2010/86820,2010,A1 Location in patent:Page/Page column 52
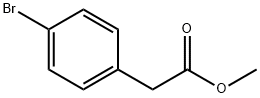
41841-16-1
215 suppliers
$5.00/5g

1236357-63-3
11 suppliers
inquiry
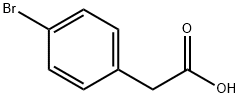
1878-68-8
489 suppliers
$6.00/10g

1236357-63-3
11 suppliers
inquiry

