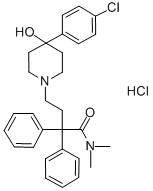
Loperamide hydrochloride synthesis
- Product Name:Loperamide hydrochloride
- CAS Number:34552-83-5
- Molecular formula:C29H34Cl2N2O2
- Molecular Weight:513.5
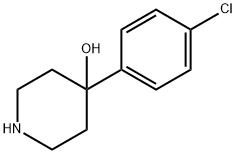
39512-49-7
314 suppliers
$24.39/1g
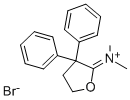
37743-18-3
78 suppliers
$60.00/2mg

34552-83-5
533 suppliers
$20.00/10mg
Yield:34552-83-5 67%
Reaction Conditions:
Stage #1: 4-(4-chlorophenyl)-4-hydroxypiperidinewith sodium carbonate;potassium iodide in 1,3-dioxolane-4-methanol; for 0.25 h;
Stage #2: N-(dihydro-3,3-diphenyl-2(3H)-furanylidene)-N-methylmethanaminium bromide in 1,3-dioxolane-4-methanol at 60; for 2 h;
Stage #3: with hydrogenchloride in 1,3-dioxolane-4-methanol;isopropyl alcohol; for 0.333333 h;
Steps:
1
Example 1 : Preparation of 4-[4-(4-diphenyl)-4-hvdroxypiperidino1-N,N- dimethyl-2,2-diphenylbutyramide hydrochloride (loperamide)0.111 g (5.2-10-4 mol, 1 eq) of 4,4-chlorophenyl-4-hydroxypiperidine, 0.062 g (5.8-10-4 mol, 1.11 eq) of sodium carbonate, 0.0009 g (0.24% by weight) of potassium iodide were weighed and dissolved in 0.5 ml_ of glycerol formal. The resulting mixture was stirred for 15 minutes. Afterwards 0.2089 g (6.03-10-4 mol, 1.15 eq) of N,N-dimethyl-(3,3-diphenyltetrahydro-2- furyliden)ammonium bromide were added and heated at 6O0C. After 2 hours, the reaction mixture was left to cool to room temperature. The crude product was centrifuged at 18000 rpm for 30 minutes at 4O0C. The two phases were separated. To the supernatant 0.65 ml_ of isopropanol saturated with hydrochloric acid (7.8-10-4 mol of HCI, 1.5 eq) was added by stirring for 20 minutes. Then, 1 mL of H2O was added by stirring for 15 minutes. After this time, it was centrifuged at 9000 rpm for 1 hour at 210C and the supernatant was removed. It was washed with water. The obtained solid was decanted and dried under vacuum. The title compound was obtained as a white solid. Yield: 67%. Rf (AcNH^Dioxane/MeOH; 20/40/40) =0.86. 1H NMR (300 MHz,CDCI3) δ 11.7 (1H, s, OH), 7.3-7.5 (14 H, m, Ar), 3 (1 H, s, CH3), 2.36 (2H, t, CH2, J=6 MHz), 2.25 (2H, t, CH2, J=6 MHz), 14.94 (4H, t, J=6 MHz).
References:
WO2008/80601,2008,A2 Location in patent:Page/Page column 10

39512-49-7
314 suppliers
$24.39/1g

37743-18-3
78 suppliers
$60.00/2mg

108-10-1
884 suppliers
$12.00/25ml

34552-83-5
533 suppliers
$20.00/10mg