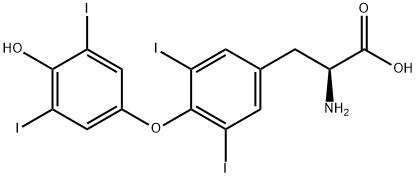
L-Thyroxine synthesis
- Product Name:L-Thyroxine
- CAS Number:51-48-9
- Molecular formula:C15H11I4NO4
- Molecular Weight:776.87
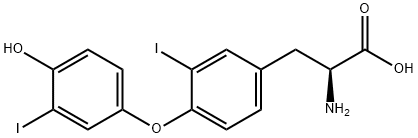
70-40-6
16 suppliers
inquiry

51-48-9
392 suppliers
$5.00/25mg
Yield:51-48-9 91.2%
Reaction Conditions:
Stage #1: 3,3′-diiodothyroninewith methylamine in methanol at 25 - 30;
Stage #2: with iodine in methanol at -8 - 0;
Stage #3: with potassium dihydrogenphosphate;sodium hydrogensulfite in methanol at 15 - 20;
Steps:
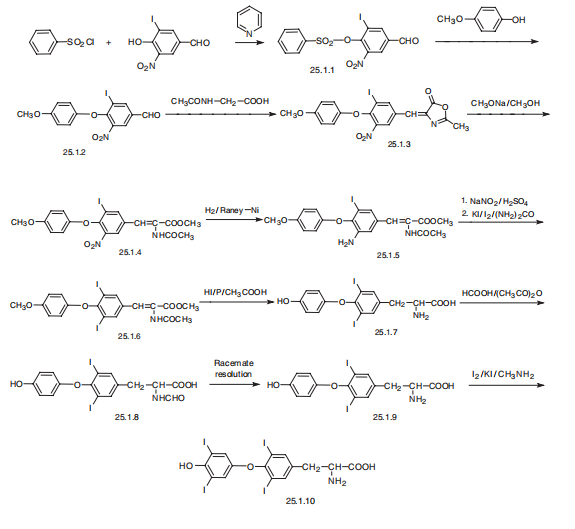
3 Example-3 Preparation of Levothyroxine
RBF was charged with lOOgms of 3,5-Diiodothyronine and l000ml of methanolic monomethylamine at 25-30°C and stined for 15-20mm to get clear solution. The reaction mixture was then cooled to -8 to 0°C, and added with Iodine solution at -8 to 0°C overperiod of 2hrs and maintained for 30-45mm at -8 to 0°C.The temperature of the reaction mixture was slowly brought to 15-20°C and charged with lOOgms of sodium bisulphite followed by 300gms of Potassium dihydrogen phosphate.The temperature of the reaction mixture was adjusted to 25-30°C and maintained for 30 - 45mm at 25-30°C. The resultant mixture was filtered and washed with 200m1 of water followed by 200m1 of acetonitrile,dried under vacuum for 12-l5hrs at 55-60°C to get l3Ogms of Levothyroxine with purity NLT 98.5% and yield of 84.5-91.2%.
References:
WO2015/11573,2015,A1 Location in patent:Page/Page column 7
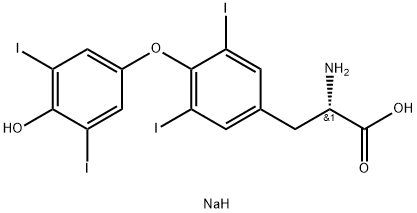
55-03-8
273 suppliers
$5.00/25mg

51-48-9
392 suppliers
$5.00/25mg
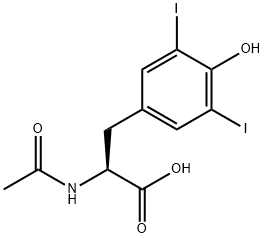
1027-28-7
170 suppliers
$12.00/5g

51-48-9
392 suppliers
$5.00/25mg
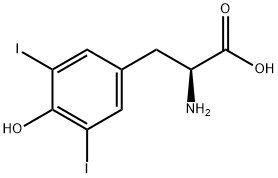
300-39-0
268 suppliers
$10.00/5g

51-48-9
392 suppliers
$5.00/25mg