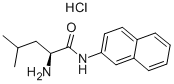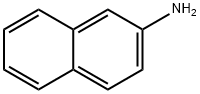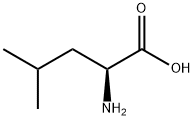
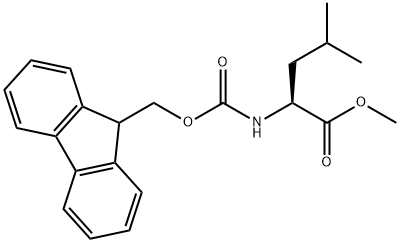
L-Leucine synthesis
- Product Name:L-Leucine
- CAS Number:61-90-5
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
139551-80-7
13 suppliers
inquiry

61-90-5
792 suppliers
$10.00/25G
Yield:-
Reaction Conditions:
Stage #1: Nα-Fmoc-Leu-OMewith dimethylsulfoxonium methylide in tetrahydrofuran at 20; for 0.333333 h;Inert atmosphere;
Stage #2: with water in tetrahydrofuran;Inert atmosphere;
Steps:
3 4.2 Reaction of N-Fmoc-α-amino acid methyl esters (1a-e) with dimethylsulfoxonium methylide (2)
General procedure: The appropriate N-Fmoc-a-amino acid methyl esters (1a-e, 1 mmol) were added to a solution of the dimethylsulfoxonium methylide (2, 2 mmol) in THF and the mixture was stirred at room temperature under inert N2 atmosphere. The reaction monitored by TLC (chloroform/methanol, 95:5 v/v) was completed after 20 min. The mixture was evaporated to dryness under reduced pressure and redissolved in 10 mL of water. The aqueous solution was extracted with diethyl ether (3 x 10 mL). The corresponding deprotected products 3a-e were recovered as N-nosyl derivatives 4a-e. To this aim the aqueous solution containing the α-amino acids (3a-e, 1 mmol) was allowed to react with nosyl chloride (1 mmol) in dioxane at room temperature for 1 h. The mixture was made basic with triethylamine (Et3N) (3 mmol). The mixture was evaporated to dryness under reduced pressure. An aqueous solution of HCl 1 N was then added and the acidified solution (pH 2) was extracted with ethyl acetate (3 x 10 mL). The organic layer was dried (Na2SO4) and evaporated to dryness under reduced pressure to give the corresponding N-nosyl derivatives 4a-e.
References:
Spinella, Mariagiovanna;De Marco, Rosaria;Belsito, Emilia L.;Leggio, Antonella;Liguori, Angelo [Tetrahedron,2013,vol. 69,# 8,p. 2010 - 2016]
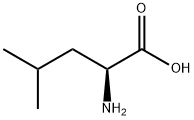
328-39-2
369 suppliers
$10.00/25g

61-90-5
792 suppliers
$10.00/25G
![L-Leucine, N-[(2-propyn-1-yloxy)carbonyl]-](/CAS/20210305/GIF/439912-47-7.gif)
439912-47-7
0 suppliers
inquiry

61-90-5
792 suppliers
$10.00/25G
![L-Leucine, N-[(1,1-dimethylethoxy)carbonyl]-, [3,4,5-tris(octadecyloxy)phenyl]methyl ester](/CAS/20210305/GIF/1258442-23-7.gif)
1258442-23-7
0 suppliers
inquiry

61-90-5
792 suppliers
$10.00/25G