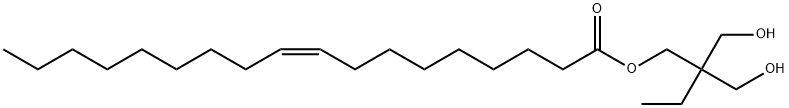
HEXAGLYCERINE MONOOLEATE synthesis
- Product Name:HEXAGLYCERINE MONOOLEATE
- CAS Number:4813-60-9
- Molecular formula:C24H46O4
- Molecular Weight:398.62
Yield:-
Reaction Conditions:
with 1.05HO3P*0.95C6H5O3P(2-)*0.1H2O*Zr(2+) at 180; for 1 h;
Steps:
2.3. Reaction procedure
General procedure: Known quantities of oleic acid (OA; C18.1; cis-9-octadecenoic acid) and polyol (glycerol or TMP) were taken in a glass, round bottom flask placed in a temperature-controlled oil bath, fitted with a water-cooled reflux condenser. To it, 5 wt% of catalyst with respect to OA was added. Temperature of the reaction mixture was raised to a desired value and the reaction was conducted for a specific period of time. At the end of the reaction, the catalyst was separated by centrifugation. The liquid product was treated with petroleum ether (20-30 ml). Two layers formed with the bottom being glycerol and the top being OA-glycerides and unconverted OA. Glycerol was separated out by decantation. Petroleum ether in the top layer portion was removed using a rotary evaporator. OA conversion was estimated by titration with 0.1 N NaOH solution and using 1, 10-phenolphthalein as indicator. The composition of OA-glycerides was determined using a PerkinElmer (Series 200) high performance liquid chromatograph fitted with an ELSD detector (from Gilson) and a reverse-phase PerkinElmer Brownlee column (C-18 Spheri-5, 250 × 4.6 mm with a 5 μm particle size). In kinetic studies, samples were withdrawn at regular intervals of time and analyzed as described above. Esterification reactions of TMP were also done with fatty acids of varying chain length [valeric acid (C5; pentanoic acid), caprylic acid (C8; octanoic acid), capric acid (C10; n-decanoic acid) and lauric acid (C12; n-dodecanoic acid)].
References:
Varhadi, Poonam;Kotwal, Mehejabeen;Srinivas [Applied Catalysis A: General,2013,vol. 462-463,p. 129 - 136]
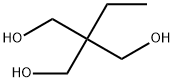
77-99-6
525 suppliers
$6.00/100g
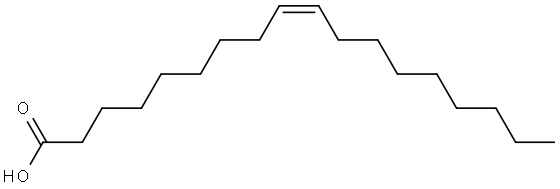
112-80-1
986 suppliers
$5.00/10g

4813-60-9
20 suppliers
inquiry

25111-05-1
1 suppliers
inquiry