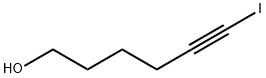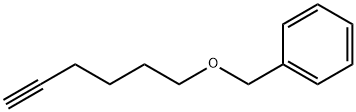
HEX-5-YNYLOXYMETHYL-BENZENE synthesis
- Product Name:HEX-5-YNYLOXYMETHYL-BENZENE
- CAS Number:60789-55-1
- Molecular formula:C13H16O
- Molecular Weight:188.27
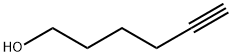
928-90-5
188 suppliers
$8.00/250mg
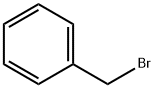
100-39-0
432 suppliers
$10.00/10g

60789-55-1
12 suppliers
inquiry
Yield:60789-55-1 99%
Reaction Conditions:
Stage #1: 5-hexyl-1-olwith sodium hydride in tetrahydrofuran at 0; for 0.5 h;
Stage #2: benzyl bromide in tetrahydrofuran at 0 - 20; for 90 h;
Steps:
29.A Step A: [(hex-5-yn-1-yloxy)methyl]benzene
To a stirred solution of 5-hexyn-1-ol (5.36 g, 54.6 mmol, 1 eq) in tetrahydrofuran (35 mL), cooled to 0 °C, was added sodium hydride (60% dispersion; 3.28 g, 81.9 mmol, 1.5 eq) portionwise and the mixture was allowed to stir for 30 min. Benzyl bromide (6.49 mL, 54.6 mmol, 1 eq) was added dropwise and the mixture was allowed to warm to ambient temperature and stir for 90 h. The reaction was cooled to 0 °C and quenched by the addition of saturated aqueous ammonium chloride (30 mL) then diluted with water (30 mL). The mixture was extracted with ethyl acetate (2 x 150 mL), and the combined organic extracts were washed with brine (100 mL), dried (magnesium sulfate) and concentrated in vacuo. Purification by automated flash column chromatography (CombiFlash Rf, 120 g RediSep silica cartridge) eluting with a gradient of 0- 10% ethyl acetate in iso-heptane afforded the desired product as a yellow oil (10.2 g, 54.2 mmol, 99%). LC/MS (C13H16O) 189 [M+H]+; RT 2.21 (LCMS-V-C) 1H NMR (400 MHz, Chloroform-d) d 7.40 - 7.24 (m, 5H), 4.45 (s, 2H), 3.44 (t, J = 6.3 Hz, 2H), 2.77 (t, J = 2.7 Hz, 1H), 2.17 (td, J = 7.0, 2.6 Hz, 2H), 1.70 - 1.57 (m, 2H), 1.56 - 1.37 (m, 2H).
References:
WO2021/18858,2021,A1 Location in patent:Page/Page column 170

100-39-0
432 suppliers
$10.00/10g

60789-55-1
12 suppliers
inquiry
100-72-1
187 suppliers
$8.00/250mg

60789-55-1
12 suppliers
inquiry

928-90-5
188 suppliers
$8.00/250mg
100-51-6
1395 suppliers
$5.00/100g
103-50-4
310 suppliers
$5.00/25g

60789-55-1
12 suppliers
inquiry