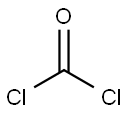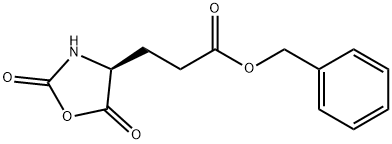
5-Benzyl L-glutamate N-carboxyanhydride synthesis
- Product Name:5-Benzyl L-glutamate N-carboxyanhydride
- CAS Number:3190-71-4
- Molecular formula:C13H13NO5
- Molecular Weight:263.25
Yield:3190-71-4 95.4%
Reaction Conditions:
in tetrahydrofuran;toluene at 50; for 1 h;
Steps:
8 L-benzylglutamate NCA
Example 8
L-benzylglutamate NCA
Vacuum-dried H-Glu(OBn)-OH (71.2 g, 300.0 mmol) was suspended in 900 mL of anhydrous THF. Phosgene (20% in toluene) (210 mL, 420 mmol) was added to the amino acid suspension at room temperature and after ten minutes, the mixture was heated to 50° C.
The amino acid dissolved over the course of approx. 1 hr, forming a clear solution.
The solution was slightly cooled and concentrated on the rotovap.
Fresh anhydrous THF (400 mL) was added to the residue and the solution was re-evaporated on the rotovap to give a colorless solid, which was dissolved in 300 mL anhydrous THF, transferred to a 4 L beaker and precipitated by the slow addition of 1.5 L of anhydrous heptane.The pure NCA was isolated by suction filtration and dried in vacuo. 75.31 g (95.4% yield) of Glu(OBn) NCA was isolated as a colorless, crystalline solid. 1H NMR (CDCl3) δ 7.36 (5H), 6.40 (1H), 5.14 (2H), 4.40 (1H), 2.60 (2H), 2.22 (2H).
References:
US2013/280306,2013,A1 Location in patent:Paragraph 0394; 0395

1676-73-9
386 suppliers
$6.00/5g

3190-71-4
161 suppliers
$8.00/250mg
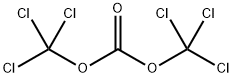
32315-10-9
414 suppliers
$10.00/1g

1676-73-9
386 suppliers
$6.00/5g

3190-71-4
161 suppliers
$8.00/250mg

1676-73-9
386 suppliers
$6.00/5g
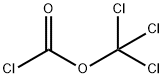
503-38-8
113 suppliers
$15.00/5g

3190-71-4
161 suppliers
$8.00/250mg
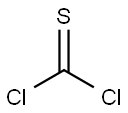
463-71-8
147 suppliers
$32.00/10g

1676-73-9
386 suppliers
$6.00/5g

3190-71-4
161 suppliers
$8.00/250mg