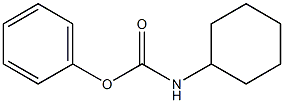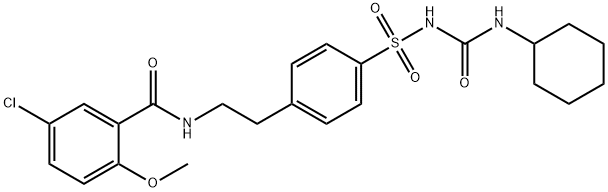
Glibenclamide synthesis
- Product Name:Glibenclamide
- CAS Number:10238-21-8
- Molecular formula:C23H28ClN3O5S
- Molecular Weight:494
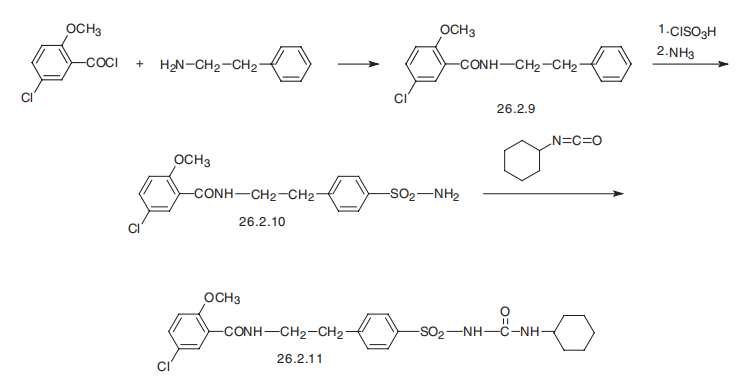
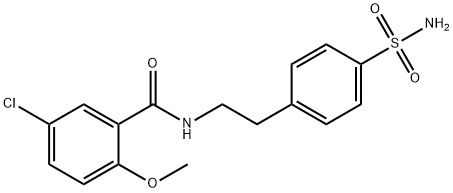
16673-34-0
184 suppliers
$18.00/1g
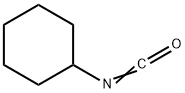
3173-53-3
237 suppliers
$24.00/25g

10238-21-8
628 suppliers
$6.00/1g
Yield:10238-21-8 4.49 g
Reaction Conditions:
with 18-crown-6 ether;potassium tert-butylate in N,N-dimethyl-formamide at 0 - 5; for 6 h;Reflux;
Steps:
1.5 Addition:
3.69 g (10 mmol) of Compound IV was dissolved in 50 mL of DMF.1.34 g (12 mmol) of potassium tert-butoxide and 0.8 g (3 mmol) of 18-crown-6 ether are added;Cyclohexyl isocyanate is dissolved in DMF,Formulated as a 1mol/L solution13 mL of this solution was added dropwise to the solution of compound IV at 0-5°C.After it is completely added,Warming to reflux reaction for 6h,Pour into 1N dilute hydrochloric acid at 0-5°C,Filtering,Vacuum drying4.49 g (9.1 mmol) of glyburide was obtained.
References:
CN107879955,2018,A Location in patent:Paragraph 0052
3438-16-2
213 suppliers
$5.00/1g

10238-21-8
628 suppliers
$6.00/1g
33924-49-1
38 suppliers
$130.00/250mg

10238-21-8
628 suppliers
$6.00/1g
64-04-0
8 suppliers
$10.00/5mL

10238-21-8
628 suppliers
$6.00/1g