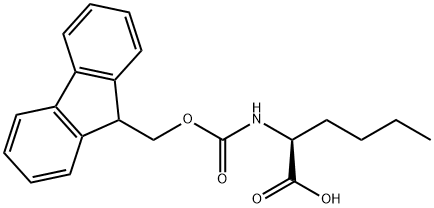
FMOC-NLE-OH synthesis
- Product Name:FMOC-NLE-OH
- CAS Number:77284-32-3
- Molecular formula:C21H23NO4
- Molecular Weight:353.41
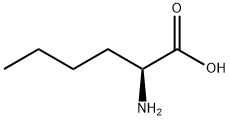
327-57-1
323 suppliers
$6.00/1g
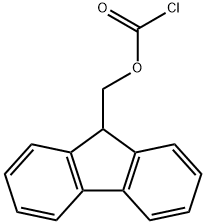
28920-43-6
533 suppliers
$5.00/5g

77284-32-3
248 suppliers
$20.00/1g
Yield:-
Reaction Conditions:
Stage #1:L-Norleucine with sodium carbonate in 1,4-dioxane;waterCooling with ice;
Stage #2:(fluorenylmethoxy)carbonyl chloride in 1,4-dioxane;water at 20; for 5 h;
Steps:
General procedure: The N-FMOC derivatizedracemic and L-amino acids and methyl esters were preparedaccording to conventional methods.22 Racemic or L-α-amino acid (5 mmol) was dissolved in 10% aqueoussodium carbonate solution (12.5 mmol). Dioxane (7.5 mL)was then added and the mixture was stirred in an ice-bath.After that, 9-FMOC chloride (5 mmol) was added slowlyand stirred at room temperature for 5 h. Now, the reactionmixture was poured into water and extracted with ether.The aqueous solution obtained was acidified with c-HCl inan ice-bath. Finally, the resulting N-FMOC α-amino acidwas filtered and dried under vacuum. In order to prepareracemic or L-FMOC α-amino acid methyl ester, the correspondingFMOC α-amino acid (1 mmol) synthesized in theprevious step was dissolved in 5 mL of anhydrous methanolwith N,N0-dicyclohexylcarbodiimide (1.1 mmol). Themixture was stirred at room temperature for 12 h, filteredand dried under vacuum to get N-FMOC α-amino acidmethyl ester.
References:
Islam, Md. Fokhrul;Adhikari, Suraj;Paik, Man-Jeong;Lee, Wonjae [Bulletin of the Korean Chemical Society,2019,vol. 40,# 4,p. 332 - 338]

28920-43-6
533 suppliers
$5.00/5g

77284-32-3
248 suppliers
$20.00/1g
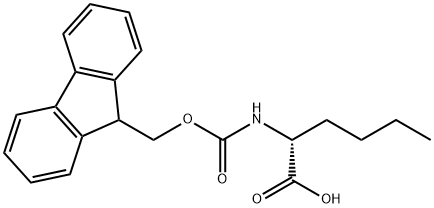
112883-41-7
129 suppliers
$7.00/250mg
616-06-8
258 suppliers
$12.00/1mg

77284-32-3
248 suppliers
$20.00/1g

112883-41-7
129 suppliers
$7.00/250mg