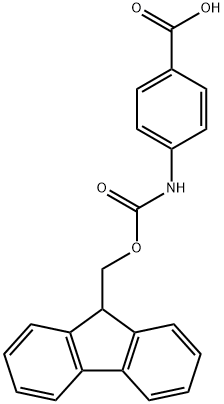
FMOC-4-AMINOBENZOIC ACID synthesis
- Product Name:FMOC-4-AMINOBENZOIC ACID
- CAS Number:185116-43-2
- Molecular formula:C22H17NO4
- Molecular Weight:359.37
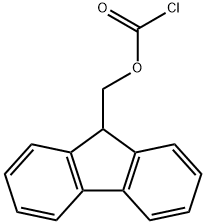
28920-43-6
530 suppliers
$5.00/5g
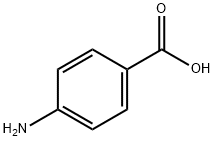
150-13-0
905 suppliers
$5.00/25g

185116-43-2
120 suppliers
$8.00/1g
Yield: 94%
Reaction Conditions:
for 24 h;Inert atmosphere;
Steps:
N--Fmoc--p--amino benzoic acid
p--Aminobenzoic acid (10 g, 73 mmol) was dissolved in dry NMP (50 ml) under an inert atmosphere followed by the dropwise addition of Fmoc--Cl (18.9 g, 73 mmol) in dry NMP (50 ml). After 24 hrs, the reaction mixture was poured slowly into 400 ml water. The colorless precipitate was collected by filtration, washed with water and dried in vacuum at 120 oC to give N-Fmoc--p--amino benzoic acid (24.8 g, 94%). mp: 215 oC (dec.) 1H--NMR: δ (300 MHz, DMSO-d6) 4.33 (t, 3J = 6.25 Hz, 1 H); 4.54 (d, 3J = 6.25 Hz, 2 H); 7.33--7.45 (m, 4 H); 7.57 (d, 3J = 7.35 Hz, 2 H); 7.76 (d, 3J = 7.35 Hz, 2 H); 7.89 (m, 4 H), 10.08 (s, 1H); 12.69 (s, 1H). 13C--NMR and DEPT: δ (300 MHz, DMSO--d6) 46.61 (+); 65.83 (--); 117.5 (+); 120.22 (+); 124.48; 125.14 (+); 127.17 (+); 127.74 (+); 130.48 (+); 140.86; 143.31; 143.74; 153.31; 167.03. IR ν (cm---1): 3344, 2970, 2887, 2660, 2544, 1709, 1673, 1610, 1592, 1526, 1511, 1411, 1311, 1282, 1221, 1052, 850, 736. RP--HPLC (min): 26.6 min. M (FD): m/z (%) = 359.1 (100); 360.1 (17.4); calc.[C22H17NO4] = 359.1
References:
Seyler, Helga;Kilbinger, Andreas [Tetrahedron Letters,2013,vol. 54,# 8,p. 753 - 756] Location in patent:supporting information
82911-69-1
620 suppliers
$7.00/25g

150-13-0
905 suppliers
$5.00/25g

185116-43-2
120 suppliers
$8.00/1g