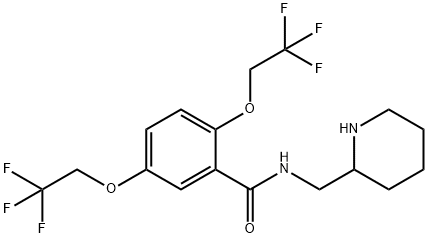
Flecainide synthesis
- Product Name:Flecainide
- CAS Number:54143-55-4
- Molecular formula:C17H20F6N2O3
- Molecular Weight:414.34
22990-77-8
224 suppliers
$27.00/5g
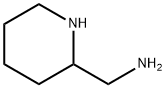
35480-31-0
49 suppliers
$19.00/1g

54143-55-4
115 suppliers
$46.00/10mg
Yield:54143-55-4 85%
Reaction Conditions:
in toluene for 10 h;Heating / reflux;
Steps:
5 Preparation of Flecainide
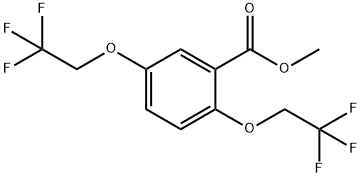
;A mixture of methyl 2,5-bis(2,2,2-trifluoroethoxy)benzoate (1.5 g), 2-(aminomethyl)piperidine (0.62 g) in toluene (3 ml) was stirred at reflux for 10 hours. After cooling to room temperature, water (10 ml) was added and two layers solution were separated. The aqueous layer was extracted with toluene (2*10 ml) and the combined organic layers were washed with water (3*10 ml). The organic layer was concentrated under reduced pressure to give Flecainide free base as a white solid (1.63 g, 85%).
References:
Wang, Zhi-Xian;Li, Yuanqiang;Guntoori, Bhaskar Reddy US2005/59825, 2005, A1 Location in patent:Page/Page column 4-5