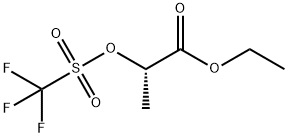
ETHYL (S)-2-(TRIFLUOROMETHYLSULFONYLOXY)PROPIONATE synthesis
- Product Name:ETHYL (S)-2-(TRIFLUOROMETHYLSULFONYLOXY)PROPIONATE
- CAS Number:84028-88-6
- Molecular formula:C6H9F3O5S
- Molecular Weight:250.19
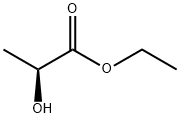
687-47-8
293 suppliers
$10.00/25g
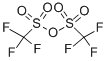
358-23-6
591 suppliers
$10.00/1g

84028-88-6
14 suppliers
$235.00/1g
Yield:84028-88-6 100%
Reaction Conditions:
with 2,6-dimethylpyridine in dichloromethane at -78 - 20; for 1.66667 h;
Steps:
7 Benzyl 2-deoxy-3-O-((R)-1′-ethoxycarbonylethyl)-4,6-O-benzylidene-2-(allyloxy-carbonylamino)-α-d-glucopyranoside (8)
1.7
Benzyl 2-deoxy-3-O-((R)-1'-ethoxycarbonylethyl)-4,6-O-benzylidene-2-(allyloxy-carbonylamino)-α-d-glucopyranoside (8)
Preparation of (-)-ethyl (S)-2-trifluromethanesulfonyl propionate:
To a solution of (-)-ethyl (S)-2-hydroxypropionate (0.23 mL, 2 mmol) in dry DCM (0.56 mL) was added 2,6-lutidine (236 μL, 2 mmol).
The reaction mixture was kept at -78 °C and Tf2O (344 μL, 2 mmol) was added dropwise, stirred for 40 min, warmed up to rt and stirred for 1 h.
The mixture was diluted with dry DCM: Hexane ((1:1) 0.7 mL) and filtered through a short silica gel column (approx. pack 1 cm of silica gel pad).
Then the reaction mixture was washed with 0.7 mL of dry DCM: Hexane (1:1) and concentrated to give (-)-ethyl (S)-2-trifluromethanesulfonyl propionate quantitatively.
To a solution of 7 (175 mg, 0.4 mmol) in dry DCM (2.6 mL) was added NaH (35 mg, 1.5 mmol, 60% oil dispersion), stirred for 1.5 h, added more NaH (10 mg, 0.4 mmol) and stirred for 1 h at rt.
The mixture was then treated with neat (-)-ethyl (S)-2-trifluromethanesulfonyl propionate dropwise and stirred for 3 h at rt.
The reaction mixture was quenched by addition of ice and extracted with CHCl3.
The organic solution was washed with saturated aq NaHCO3 and brine, dried and concentrated.
The residue was purified by column chromatography (CHCl3/EtOAc 7:0.5) to give 8 as a white solid (145 mg, 68%). _
References:
Enugala, Ramu;Pires, Marina J.D.;Marques, M. Manuel B. [Carbohydrate Research,2014,vol. 384,p. 112 - 118]