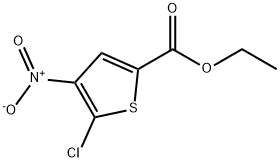
Ethyl 5-chloro-4-nitrothiophene-2-carboxylate synthesis
- Product Name:Ethyl 5-chloro-4-nitrothiophene-2-carboxylate
- CAS Number:89640-03-9
- Molecular formula:C7H6ClNO4S
- Molecular Weight:235.64
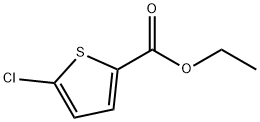
5751-82-6
144 suppliers
$9.00/1g

89640-03-9
39 suppliers
$13.00/250mg
Yield:89640-03-9 67%
Reaction Conditions:
with sulfuric acid;nitric acid at 0 - 20;
Steps:
41.A
Ethyl 5-chlorothiophene-2- carboxylate (2.7 g, 14.1 mmol) was added in portions to concentrated sulfuric acid (5 mL) and the stirred solution was cooled to below 0 0C with a methanol/ice bath. Fuming nitric acid (1.78 g, 1.2 mL, 28.3 mmol) was added slowly, keeping the temperature below 0 0C throughout the addition. On completion of addition the stirred mixture was removed from the cold bath and warmed to ambient temperature for 2 hours. The reaction was quenched by addition to ice/water (100 mL) resulting in formation of a sticky solid. The product was extracted into dichloromethane (2 x 100 mL). The combined organic extracts were dried (MgSO4), filtered and the solvent was removed to afford an orange oil. The crude material was purified by silica gel chromatography using a 95:5 mixture of hexane and ethyl acetate as eluent to afford the title product as a solid (2.23 g, 67% yield). 1H NMR (CDCl3) δ: 8.14 (IH, s), 4.37 (2H, q, J=7.11 Hz), 1.37 (3H, t, J=7.11 Hz).
References:
WO2010/114881,2010,A1 Location in patent:Page/Page column 43-44