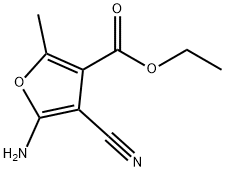
ETHYL 5-AMINO-4-CYANO-2-METHYL-3-FUROATE synthesis
- Product Name:ETHYL 5-AMINO-4-CYANO-2-METHYL-3-FUROATE
- CAS Number:14476-67-6
- Molecular formula:C9H10N2O3
- Molecular Weight:194.19
Yield:14476-67-6 90%
Reaction Conditions:
with sodium ethanolate in ethanol at -10 - 10;Large scale;
Steps:
1 Step 1. Ethyl 5-amino-4-cyano-2-methylfuran-3-carboxylate
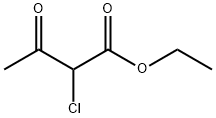
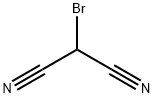
Sodium ethoxide (2.49 L, 21 %w/w, 6.68 mol) was diluted with ethyl alcohol (3.00 L). The jacket temperature was set to - 10°C. Separately, a solution of ethyl 2-chloro-3-oxobutanoate (840 mL, 6.08 mol) and malononitrile (401 g, 6.08 mol) were taken up in ethanol (2.00 L). The substrate solution was added, maintaining the internal temperature below 10°C. Upon complete addition, the jacket temperature was adjusted to 10°C and the slurry was stirred overnight. A total of 2.90 L of solvent was removed by vacuum distillation. The temperature was then ramped to 45°C and water (10 L) was charged to the reactor. The slurry was stirred overnight and cooled to 11°C. The solid was collected by vacuum filtration and then slurried/washed with additional water (10 L). The solid was air dried to afford the title product (1.058 kg, 90%). 1H NMR (400 MHz, DMSO-d6) δ 7.42 (s, 2H), 4.21 (q, J = 7.1 Hz, 2H), 2.37 (s, 3H), 1.26 (t, J = 7.2 Hz, 3H). [M+H] = 195.
References:
WO2019/104285,2019,A1 Location in patent:Page/Page column 116